Figure 1.
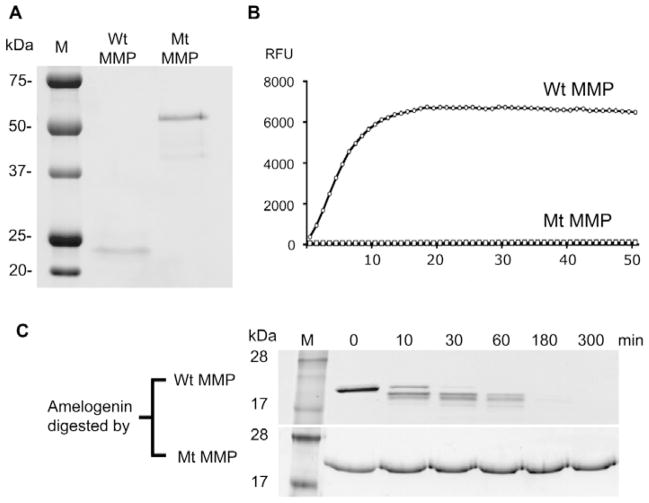
Characterizations of inactive E227A mutated rhMMP20. (A) SDS-PAGE analysis of rhMMP20s after purification showed that the 55-kDa intact protein of wild-type active rhMMP20 (left lane) was absent; however, the autolytic products of rhMMP20 were predominantly located at 25 and 21 kDa. A predominant 55-kDa band with minor smaller fragments of inactive E227A rhMMP20 can be seen in the right lane of the SDS-PAGE gel. M, protein standard; Wt MMP, active wild-type rhMMP20; Mt MMP, inactive E227A rhMMP20. (B) Quenched-peptide digestion assay showed that wild-type rhMMP20 (Wt MMP20) was active. However, the enzymatic activity of inactive E227A rhMMP20 (Mt MMP20) was undetectable. The RFU readings depended on the amount of fluorophores released from the quencher in the quenched peptide by rhMMP20 hydrolysis. RFU, relative fluorescence units. (C) SDS-PAGE analysis showed that the wild-type human recombinant amelogenin is digested by wild-type active rhMMP20 (Wt MMP20, upper row), but is not degraded by mutated inactive E227A rhMMP20 (Mt MMP, lower row).