Figure 3.
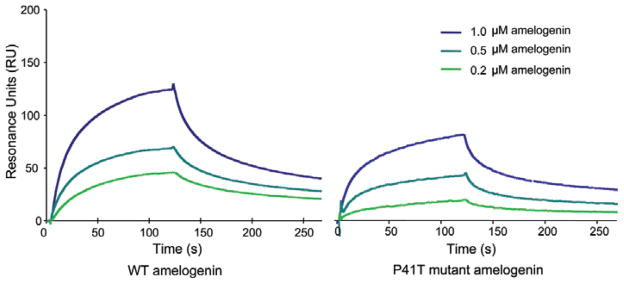
Sensorgrams of surface plasmon resonance (SPR) analysis of the interactions between inactive E227A rhMMP20 and amelogenins (wild-type rh174 or P41T mutant). The binding affinity of the immobilized E227A rhMMP20 for wild-type rh174 amelogenin (left panel) was higher than that for the P41T mutant (right panel) at each concentration (0.2, 0.5, or 1 μM). Triplicate samples were performed at each amelogenin concentration, and the average values were used for construction of sensorgrams, from which Kon (on-rate constant), Koff (off-rate constant), Ka (affinity constant), and their variability were calculated (Table).
