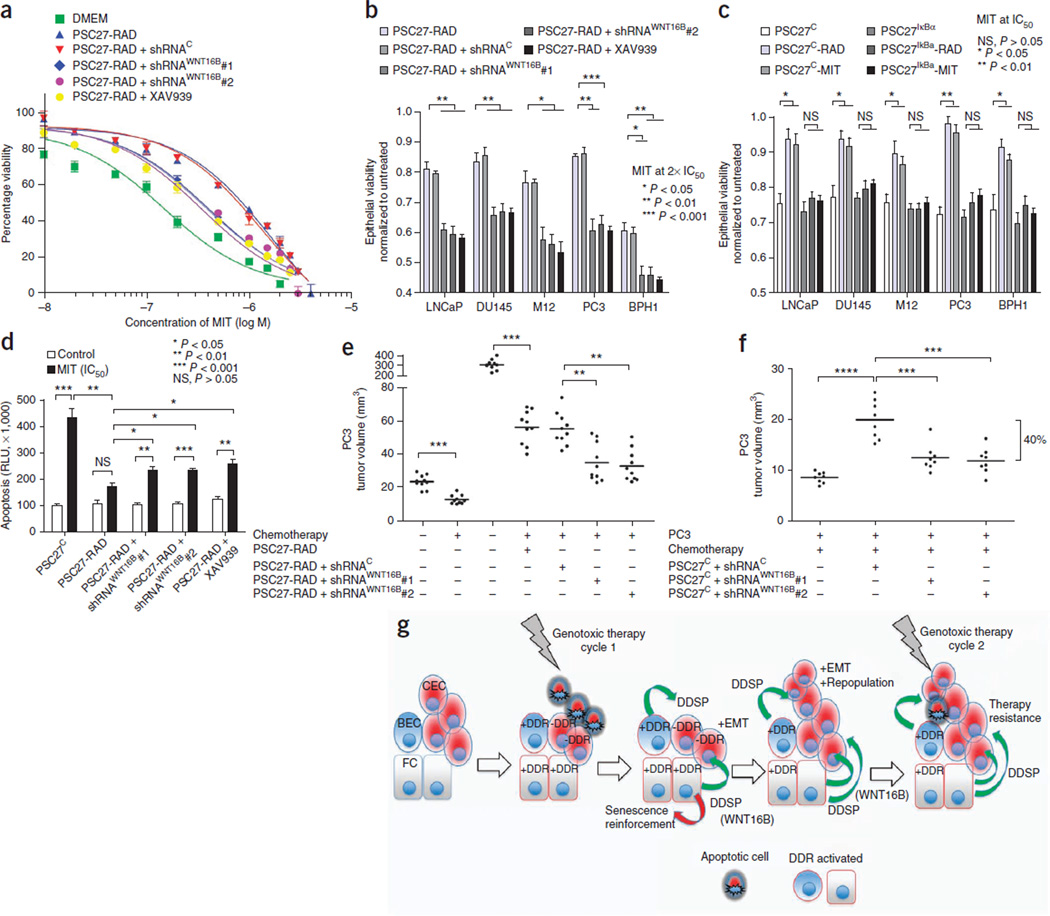
Figure 6.
Chemotherapy resistance promoted by damaged fibroblasts is attenuated by blocking WNT16B, β-catenin or NF-κB signaling. (a) Viability of prostate cancer cells across a range of MIT concentrations with (PSC27-RAD+shRNAWNT16B) or without (PSC27-RAD+shRNAC) the suppression of WNT16B in irradiated-fibroblast–conditioned medium or with the addition of the β-catenin pathway inhibitor XAV939. Data are mean ± s.e.m. of triplicates. (b) Viability of prostate cancer cells 3 d after treatment with two times the IC50 of MIT in the context of conditioned medium from irradiated prostate fibroblasts (PSC27-RAD) expressing shRNAs targeting and suppressing WNT16B (shRNAWNT16B), a vector control (shRNAC) or combined with the β-catenin pathway inhibitor XAV939. (c) Viability of prostate cancer cells 3 d after treatment with the IC50 of MIT in the context of conditioned medium from prostate fibroblasts pretreated with radiation (PSC27-RAD) or MIT (PSC27-MIT) and with (PSC27IκBα) or without (PSC27C) the suppression of NF-κB signaling. (d) Acute tumor cell responses to chemotherapy in vitro. Quantification of apoptosis by caspase 3 and 7 activity measured 24 h after the exposure of PC3 cells to vehicle or the IC50 of MIT. Data for b, c and d are mean ± s.e.m. of triplicates, and P values were determined by ANOVA followed by t test. (e,f) In vivo effects of MIT chemotherapy in the context of suppressing the induction of the expression of fibroblast WNT16B. Tumors comprised PC3 cells in combination with irradiated (PSC27-RAD) fibroblasts (e) or unirradiated (PSC27C) (f) prostate fibroblasts expressing shRNAs targeting WNT16B (shRNAWNT16B) or a vector control (shRNAC). MIT was administered every 2 weeks for three cycles, and grafts were harvested and tumor volumes determined 1 week after the final treatment. Each data point represents an individual xenograft. Tumor volumes of PSC27C+shRNAC grafts in f averaged 20 mm3, and tumor volumes of PSC27C+shRNAWNT16B grafts averaged 12 mm3 (P < 0.001). Horizontal lines are group means, with n = 10 in e and n = 8 in f. P values were determined by ANOVA followed by t test. The bracket boundaries in f are the group means for PSC27C+shRNAC grafts compared to PSC27C+shRNAWNT16B grafts showing a 40% difference in size. Asterisks, as for the previous panel. (g) Model for cell nonautonomous therapy-resistance effects originating in the tumor microenvironment in response to genotoxic cancer therapeutics. The initial round of therapy engages an apoptotic or senescence response in subsets of tumor cells and activates a DNA damage response (DDR) in DDR-competent benign cells (+DDR) comprising the tumor microenvironment. The DDR includes a spectrum of autocrine- and paracrine-acting proteins that are capable of reinforcing a senescent phenotype in benign cells and promoting tumor repopulation through progrowth signaling pathways in neoplastic cells. Paracrine-acting secretory components such as WNT16B also promote resistance to subsequent cycles of cytotoxic therapy. CEC, cancer epithelial cell; BEC, benign epithelial cell; FC, fibroblast cell; –DDR, DDR-incompetent benign cells.