Abstract
While a number of diagnostic methods have been developed, endoscopic ultrasound (EUS) still takes the most important role in the preoperative evaluation of esophageal cancer. EUS can detect lesions of all esophageal cancer and can accurately perform T staging. In a recent meta-analysis of EUS in esophageal cancer, the sensitivity and specificity of EUS on esophageal cancer were 81.6% and 99.4% in T1, 81.4% and 96.3% in T2, 91.4% and 94.4% in T3, and 92.4% and 97.4% in T4, respectively. The use of EUS can reduce unnecessary surgeries and lead to apply proper treatments to patients. The advance of endoscopic submucosal dissection have necessitated the presurgical detection of early cancer lesions without lymph node metastasis. Understanding the practical meanings of images shown by EUS is important to decide patients for whom endoscopic treatments can be effective. In early gastric cancer, EUS can accurately predict mucosal and SM1 (invasion into the submucosal layer of less than 500 µm from muscularis mucosa) lesions, which are considered as good indications for endoscopic treatments.
Keywords: Endosonography, Early gastric cancer, Esophageal neoplasms
INTRODUCTION
The development of endoscopic equipment and the national cancer screening program to discover gastrointestinal malignancy has increased markedly the diagnoses of early cancers and precancerous lesions. Endoscopic submucosal dissection (ESD) and endoscopic mucosal resection have made an innovative advancement in the treatment of early cancers without lymph node metastasis as an effective and safe treatment method.
The most effective treatment for esophageal cancer is the complete resection of its lesions through surgery. However, this cancer is often diagnosed as advanced state, making the surgical approach difficult in many cases. In addition, the 5-year survival rate of patients who undergo surgery is not high. Esophageal cancer surgeries have relatively high levels of morbidity and mortality, which raises the need to screen patients in need of surgery through a preoperative evaluation. Although a number of diagnostic methods have been developed, endoscopic ultrasonography (EUS) still takes the most important role in the preoperative evaluation of esophageal cancer. The number of patients with early gastric cancer and its precancerous lesions has increased markedly, and the advance of ESD has necessitated the presurgical detection of early cancer lesions without lymph node metastasis.
ESOPHAGEAL CANCER
Characteristics of esophageal cancer and the role of EUS
Esophageal cancer consists of squamous cell carcinoma and adenocarcinoma, and their incidence is similar across Western countries. In Korea, squamous cell carcinoma accounts for over 90% of esophageal cancer, while the incidence of adenocarcinoma is on the rise. Squamous cell carcinoma and adenocarcinoma differs from each other in terms of their causes, the location of lesion, and the pattern of lymph node metastasis, which, in turn, results in differences in their prognosis. However, there are few studies that reported esophageal cancer divided into the two types.
The most significant characteristic of esophageal cancer is that preoperative staging is difficult because superficial esophageal cancer can commonly invade into the lymph nodes around cervical and celiac axis without regional lymph node metastasis. In addition, this cancer has high levels of morbidity and mortality following the surgery, along with large differences in surgical outcome of individual hospitals. Therefore, the application of proper treatments to patients is problematic. EUS is the most important test in examining the depth of invasion and lymph node metastasis in esophageal cancer. The test can predict the lesions to perform endoscopic treatments and draw up treatment plans by distinguishing between patients who need surgical approach and chemoradiation therapy. EUS can assess the depth of the invasion of esophageal cancer and accurately diagnose patients suspected of metastatic lymph node by performing EUS guided fine needle aspiration or biopsy.1
Importance of the assessment of invasion depth
The esophageal wall anatomically consists of mucosal, submucosal, muscularis propria, and adventitial layers. In general, EUS shows the wall in five or seven layers. The first layer consists of epithelium and interface echo, and is shown to be hyperechoic. The second layer is the hypoechoic and means lamina propria and muscularis mucosa. The third layer is the hyperechoic which is submucosa. The fourth layer, muscularis propria, is the hypoechoic. The fifth layer is the hyperechoic and means adventitial layer. Sometimes a hyperechoic layer appears in the middle of the proper muscular layer. As the midlayer divides the proper muscular layer into the inner circular muscle and the outer longitudinal muscle, the esophageal wall is exhibited in a total of seven layers. On high-frequency ultrasound tests at 20 MHz or above, the epithelium appears to be divided into hyperechoic and hypoechoic layers, and the lamina propria layer is shown to be hyperechoic. Therefore, a total of nine layers can be observed.
The prognosis of esophageal cancer without lymph node metastasis is associated with T stages, pathological findings, cell differentiation, and the location of tumors. Most patients diagnosed preoperatively with T2-T3N0Mo had lymph node metastasis in the surgical result.2 Over 50% of the lesions with stage T2 in surgical findings exhibit lymph node metastasis. 3 Therefore, if preoperative evaluation can accurately predict T stages, this can greatly help assess the prognosis and make appropriate treatment plans.
Superficial esophageal cancer
Regardless of lymph node metastasis, esophageal cancers that are limited to the mucosal and submucosal layers are referred to as superficial esophageal cancer. In the tumor, tumor-nodes-metastasis (TNM) classification, superficial esophageal cancers are divided into T1s (high grade dysplasia), T1a (invasion into the muscularis mucosa), and T1b (invasion into the submucosa). Evaluating the risk of lymph node metastasis according to the depth of tumor invasion is necessary to predict prognosis and decide treatment method.
The mucosal layer is further divided into M1 (limited to the epithelial layer), M2 (invasion into the lamina propria), and M3 (invasion into the muscularis mucosa). The submucosal layer is divided into SM1 (superficial 1/3), SM2 (middle 1/3), and SM3 (deep 1/3).4,5 These divisions are useful for screening the lesions that are available for endoscopic resection in treating esophageal cancer. As most of the M1 and M2 lesions do not show lymph node metastasis, they can be treated only through local resection. Moreover, given that lesions limited to the submucosal or proper muscular layers may undergo surgical treatment as the primary option, a proper evaluation on the invasion depth of lesions is of great importance.
Usefulness of EUS in assessing T staging
The treatment method of esophageal cancer should be decided through preoperative evaluation. In terms of evaluating the depth of tumor invasion, the accuracy levels of computed tomography (CT) and positron emission tomography are significantly low. EUS can detect the lesions for all esophageal cancer and accurately perform T staging. Puli et al.6 reported that the sensitivity and specificity of EUS on esophageal cancer marked 81.6% and 99.4% in T1, 81.4% and 96.3% in T2, 91.4% and 94.4% in T3, and 92.4% and 97.4% in T4, respectively. Moreover, EUS were more accurate in advanced cancers over early cancers. In general, T1 and T2 exhibited more cases of overstaging, whereas T3 and T4 showed more cases of understaging. 6
EUS is a highly important test in deciding surgical treatments as it is proved to be highly effective in distinguishing T1 and T2 lesions from T3 and T4 lesions. Compared to evaluations based on other tests such as CT, EUS resulted in treatment method changes in 44%.7 Patients with T4 can be divided into T4a (resectable) and T4b (irresectable) according to the seventh TNM classification. Patients with T4 diagnosed by EUS have survival time less than 1 year regardless of whether or not they undergo surgery.8 Thosani et al.9 performed a meta-analysis on 19 studies regarding early esophageal cancer, and reported that the sensitivity and specificity were 85% and 87% in T1a, respectively, and 86% both in T1b. An interesting fact in this report is that the analysis was performed by dividing the sample studies into those reported in Japan and other countries. While the studies reported in Japan exhibited high levels of sensitivity and specificity at 87% and 95%, respectively, the other studies showed low levels of sensitivity and specificity at 84% and 68%m, respectively. In general, EUS for early esophageal cancer are performed using miniprobe. In this analysis, radial EUS tests yielded similar results to those when using miniprobes.9
The analysis on surgical treatments of 190 Korean early esophageal cancer revealed that lesions that invaded the lamina propria of the esophagus did not exhibit lymph node metastasis when its size was less than 3 cm.10 In order to evaluate the mucosal layer by further segmenting it through, high frequency EUS at 20 MHz or above is necessary. In addition, various methods using balloons, jellies, and condoms have been developed to acquire good quality of images. The diagnosis rate of EUS was 81% in M1 and M2, 60% in M3 and SM1, and 87% in SM2 and SM3. While their accuracy in terms of distinguishing among M1, M2, and M3 was reported to range from 81% to 100%, it had large differences among individual observers.1
EARLY GASTRIC CANCER
Endoscopic treatments of early gastric cancer
In treating early gastric cancer, ESD is regarded as the most effective and safe treatment. Increased experiences in ESD and the development of various supplementary equipment have allowed the removal of lesions regardless of their size or location. The lesions that can be removed with ESD are those that do not invade deeply into the submucosal layer and without lymph node metastasis. While imaging test methods have been developed, diagnostic methods to predict the lymph node metastasis accurately in gastric cancer are not available to date.
The absolute indications of ESD include lesions limited to the mucosa with well differentiation, elevated lesions less than 2 cm, and depressed lesions less than 1 cm. Mucosal cancer arising from adenomas are the lesion for which endoscopic treatments can be effective regardless of their size. Very old patients (>75 years old) with high surgical risks and patients with severe underlying diseases can use the expanded indications of ESD. Therefore, preoperative evaluation should identify the lesions that have penetrated into the submucosal layer of less than 500 µm.
Application of EUS in assessing T staging
Early gastric cancer can have a survival rate of over 90% through surgical treatment and chemotherapy. The preoperative detection of lesions that have invaded within the submucosal layer is essential to predict the prognosis of patients and establish surgical plans. On the other hand, when evaluating the invasion depth of lesions only through endoscopic findings, the limitations exist in diagnostic accuracy.
Puli et al.11 reported a meta-analysis of 22 studies that evaluated the usefulness of EUS in gastric cancer staging, which were undertaken from 1993 to 2006. The results showed that the sensitivity and specificity were 88.1% and 100% in T1, 82.3% and 95.6% in T2, 89.7% and 94.7 in T3, and 99.2% and 96.7% in T4. In deciding T stages, EUS turned out to be more accurate in advanced gastric cancers compared to early cancers.11 Mocellin et al.12 analyzed 5,601 patients in 54 studies. As a result, EUS was found to be particularly accurate in distinguishing between T3 to 4 and T1 to 2 lesions, with the sensitivity and specificity of 86% and 91%, respectively.12
Understanding the practical meanings of images shown by EUS is important to decide patients for whom endoscopic treatments can be effective (Fig. 1). Mouri et al.13 evaluated the usefulness of EUS in examining the adaptation to endoscopic treatments and invasion depth for early gastric cancer. Ninetynine percentage of EUS-M (lesions limited to the first and second layers based on EUS) and 87% of EUS-M/SM border (lesions with change in the third layer on EUS, but not deeper than 1 mm) were pathologically mucosal and SM1 (invasion into the submucosal layer of less than 500 µm from muscularis mucosa) lesions. Ninety-one percentage of EUS-SM (1 mm or deeper invasion of the third layer based on EUS) were diagnosed as SM2 lesions (invasion into the submucosal layer of 500 µm or above from muscularis mucosa). For the EUS-M and EUS-M/SM border, tumor cells were not discovered in the vertical resection margin in all patients who underwent endoscopic resection. Therefore, these are considered as good indications for endoscopic treatments.13
Fig. 1.
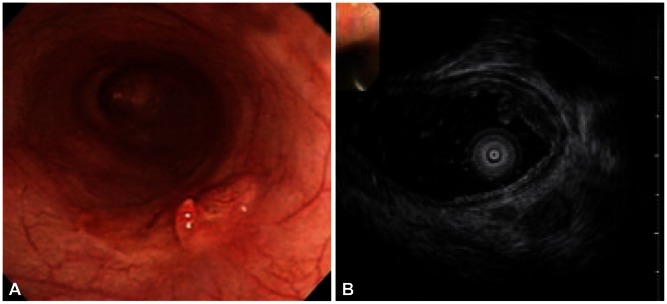
Esophageal carcinoma with submucosal invasion. (A) Endoscopy shows flat nodular elevated mass. (B) Hypoechoic mass in the mucosal layer with the third layer thinning is seen on endoscopic ultrasonography.
Limitations of the application of EUS
The EUS should be performed on all esophageal cancer patients in the preoperative evaluation.14 The use of EUS can reduce unnecessary surgeries and lead to applying proper treatments to patients. However, despite various positive research results, this test has certain limitations. Firstly, the range of esophageal cancer cannot be accurately evaluated through endoscopy only. The range of lesions exhibited by chromoendoscopy is mostly larger and more multifocal. As EUS-based evaluations on the depth of invasion should be performed based on the lesions exhibited on endoscopic images, an accurate evaluation of all the areas of multifocal scattered lesions may be difficult. EUS are also limited when applying to large lesions with esophageal obstruction. Sometimes, patients in whom the obstructing tumor area was dilated might experience perforation. The use of water or jellies is limited in the cervical esophagus due to the risk of aspiration pneumonia, which causes difficulties evaluating superficial esophageal cancer. If EUS diagnoses result in overstaging or understaging, patients who can be treated with endoscopic method might have to undergo unnecessary surgeries. This is caused by fibrosis due to esophagitis, poor layer structures due to biopsy, and the hyperplasia of lymph follicles and cellular infiltration around tumors.
The practice of EUS before ESD in early gastric cancer patients may differ by hospitals. The EUS is useful in patients with gastric adenoma or mucosal cancer who are suspected to have invasion into the submucosal layer on endoscopy. In particular, for cancer located in the regions that are expected to take long to resect endoscopically due to the difficulty of performing the ESD, the EUS can be an effective option to accurately evaluate the depth of invasion and reduce unnecessary procedures. In case of large lesions, EUS can predict the regions where blood vessels are developed abundantly and approach the region accordingly, as well as predict bleeding. However, in the lesions with ulcer or fibrosis, the accuracy of the depth of invasion is low.
Footnotes
The author has no financial conflicts of interest.
References
- 1.Kim HG, Moon JS, Cho JW. Korean EUS Study Group, Korean Society of Gastrointestinal Endoscopy, editors. Textbook of Endoscopic Ultrasonography. 1st ed. Seoul: Jin; 2011. Esophagus & mediastinum; pp. 34–63. [Google Scholar]
- 2.Stiles BM, Mirza F, Coppolino A, et al. Clinical T2-T3N0M0 esophageal cancer: the risk of node positive disease. Ann Thorac Surg. 2011;92:491–496. doi: 10.1016/j.athoracsur.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Rice TW, Zuccaro G, Jr, Adelstein DJ, Rybicki LA, Blackstone EH, Goldblum JR. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg. 1998;65:787–792. doi: 10.1016/s0003-4975(97)01387-8. [DOI] [PubMed] [Google Scholar]
- 4.Endo M, Yoshino K, Kawano T, Nagai K, Inoue H. Clinicopathologic analysis of lymph node metastasis in surgically resected superficial cancer of the thoracic esophagus. Dis Esophagus. 2000;13:125–129. doi: 10.1046/j.1442-2050.2000.00100.x. [DOI] [PubMed] [Google Scholar]
- 5.Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg. 2011;253:271–278. doi: 10.1097/SLA.0b013e3181fbad42. [DOI] [PubMed] [Google Scholar]
- 6.Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479–1490. doi: 10.3748/wjg.14.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gheorghe C, Stanescu C, Gheorghe L, et al. Preoperative noninvasive EUS evaluation in patients with esophageal cancer considered for esophagectomy. J Gastrointestin Liver Dis. 2006;15:137–141. [PubMed] [Google Scholar]
- 8.Fockens P, Kisman K, Merkus MP, van Lanschot JJ, Obertop H, Tytgat GN. The prognosis of esophageal carcinoma staged irresectable (T4) by endosonography. J Am Coll Surg. 1998;186:17–23. doi: 10.1016/s1072-7515(97)00131-2. [DOI] [PubMed] [Google Scholar]
- 9.Thosani N, Singh H, Kapadia A, et al. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75:242–253. doi: 10.1016/j.gie.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Choi JY, Park YS, Jung HY, et al. Feasibility of endoscopic resection in superficial esophageal squamous carcinoma. Gastrointest Endosc. 2011;73:881–889. doi: 10.1016/j.gie.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Puli SR, Batapati Krishna Reddy I, Bechtold ML, Antillon MR, Ibdah JA. How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:4011–4019. doi: 10.3748/wjg.14.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: a meta-analysis. Gastrointest Endosc. 2011;73:1122–1134. doi: 10.1016/j.gie.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Mouri R, Yoshida S, Tanaka S, Oka S, Yoshihara M, Chayama K. Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. J Clin Gastroenterol. 2009;43:318–322. doi: 10.1097/MCG.0b013e3181775966. [DOI] [PubMed] [Google Scholar]
- 14.Kim EY. Introduction: value of endoscopic ultrasound-guided fine needle aspiration. Clin Endosc. 2012;45:115–116. doi: 10.5946/ce.2012.45.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]


