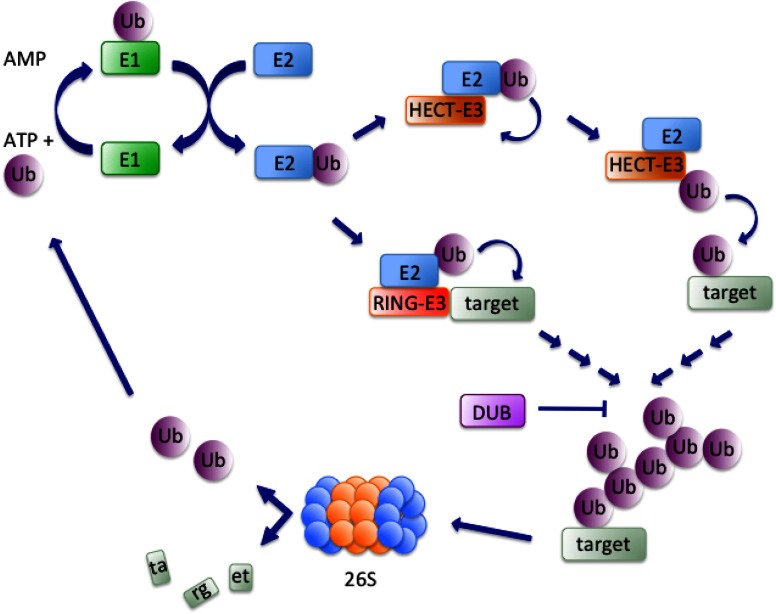
Figure 1.
Simplified scheme of ubiquitylation. The ubiquitylation cascade initiates with an ATP-dependent reaction consisting in the formation of a thiolester bond between a cysteine in the active site of the E1-activating enzyme and G76 in Ubiquitin (Ub). Next, Ubiquitin is transferred to the active cysteine of an E2-conjugating enzyme that interacts with an E3-ligase. The latter can either directly transfer Ubiquitin to lysine residues of the acceptor substrate, as is the case for HECT-E3s, or recruit substrates to the E2 enzyme, a mechanism that characterizes RING-E3s. Finally, ubiquitylated substrates are shuttled to the 26S proteasome and Ubiquitin is recycled for another round of reactions. DUBs oppose substrate degradation by reversing the process of ubiquitylation.