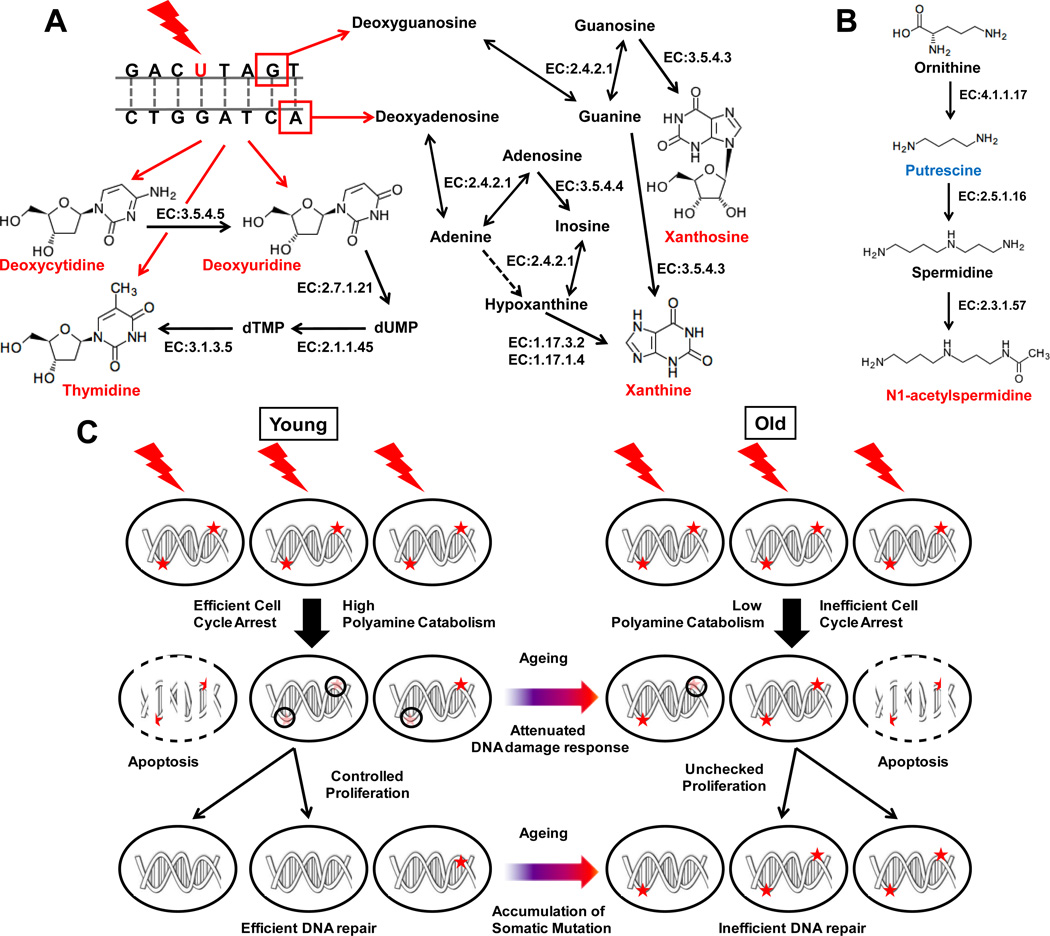
Figure 7. Biochemical pathway involved in production of radiation exposure biomarkers related to (A) nucleic acid metabolism and (B) polyamine metabolism.
Quantitatively elevated and depleted metabolites are shown in red and blue, respectively, while grey metabolites indicate no change or not measured in this study. Possible chemical modification of DNA contributing to production of these metabolites is shown with red bases indicating base modification (such as C→U) and red boxes indicating nucleotide excision sites following single-strand breaks. The enzymes involved in these metabolic pathways such as cytidine deaminase (EC 3.5.4.5), thymidine kinase (EC 2.7.1.21), thymidylate synthase (EC 2.1.1.45), 5',3'-nucleotidase (mitochondrial) (EC 3.1.3.5), purine-nucleoside phosphorylase (EC 2.4.2.1), guanine deaminase (EC 3.5.4.4), adenine deaminase (EC 3.5.4.3), xanthine dehydrogenase (EC 1.17.3.2), xanthine oxidase (EC 1.17.1.4), ornithine decarboxylase (EC 4.1.17), spermidine synthase (EC 2.5.1.16) and spermidine/spermine N1-acetyltransferase (EC 2.3.1.57) are indicated next to corresponding steps (shown by solid grey arrow). Lines with arrows in both directions for purine-nucleoside phosphorylase indicate its ability to catalyze ribosyltransferase activity in both directions. Dotted grey arrow indicates that the corresponding enzymatic activity has not been reported in mice. (C) Putative mechanism showing the role of polyamine catabolism in radiation-induced damage-repair through cell cycle arrest and the effect of aging in the process. Dark red asterisk indicate DNA damage lesion caused by radiation exposure that would cause mutation if replicated without repair during proliferation. Faint red asterisk encircled by black circles indicate recruitment of DNA-damage repair machinery during cell cycle arrest leading to successful DNA damage repair. Attenuation of polyamine catabolism in older mice may lead to inefficient arrest of cell cycle and, thus, impairment of DNA damage repair and propagation of mutation in proliferating somatic cells.