Scheme 1.
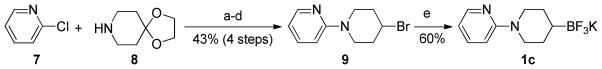
Preparation of potassium N-(2-pyridinyl)-4-trifluoroboratopiperidine 1c
Reactions conditions: a) 7 (1.0 equiv), 8 (1.2 equiv), PEPPSI-IPr (2 mol %), t-BuOK (1.5 equiv), DME, rt, 24 h, quant.; b) TsOH (20 mol %), H2O/acetone:1/3, 150 °C (MW), 1 h, 83%; c) NaBH4 (1.1 equiv), MeOH, rt, 1 h, 90%; d) HBr (48% in H2O, 17 equiv), 100 °C, 14 h, 58% (81% brsm); e) B2pin2 (1.5 equiv); CuI (10 mol %), PS-PPh3 (13 mol %), MeOLi (2.0 equiv), DMF, rt, 20 h then sat. aq KHF2 (4.0 equiv), THF, rt, 2 h, 60%.
