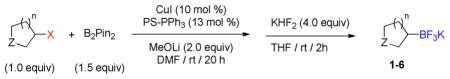
Table 1.
Borylation of Various Heterocyclic Halidesa
| |||
|---|---|---|---|
| entry | alkyl halide | product | yield (%) |
| 1 |
|
1a |
72 |
| 2 |
|
1b |
51 |
| 3 |
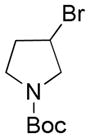
|
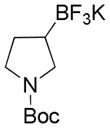 2 |
59 |
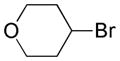
| 4 |
|
3 |
54 |
| 5 |
|
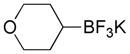 4a |
27 |
| 6 |
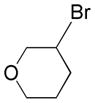
|
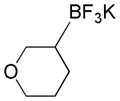 4a |
52 |
| 7 |
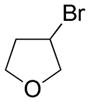
|
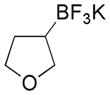 5 |
52 |
| 8 |
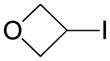
|
6 |
30 |
Reaction conditions: Halide (1.0 equiv), B2pin2 (1.5 equiv), CuI (10 mol %), PS-PPh3 (13 mol %), MeOLi (2.0 equiv), DMF ([halide] = 0.2 M), rt, 20 h then sat. aq KHF2 (4.0 equiv), THF, rt, 2 h.
