Abstract
A historically unprecedented mountain pine beetle (MPB) outbreak affected western Montana during the past decade. We examined radial growth rates (AD 1860–2007/8) of co-occurring mature healthy and MPB-infected ponderosa pine trees collected at two sites (Cabin Gulch and Kitchen Gulch) in western Montana and: (1) compared basal area increment (BAI) values within populations and between sites; (2) used carbon isotope analysis to calculate intrinsic water-use efficiency (iWUE) at Cabin Gulch; and (3) compared climate-growth responses using a suite of monthly climatic variables. BAI values within populations and between sites were similar until the last 20–30 years, at which point the visually healthy populations had consistently higher BAI values (22–34%) than the MPB-infected trees. These results suggest that growth rates two–three decades prior to the current outbreak diverged between our selected populations, with the slower-growing trees being more vulnerable to beetle infestation. Both samples from Cabin Gulch experienced upward trends in iWUE, with significant regime shifts toward higher iWUE beginning in 1955–59 for the visually healthy trees and 1960–64 for the MPB-infected trees. Drought tolerance also varied between the two populations with the visually healthy trees having higher growth rates than MPB-infected trees prior to infection during a multi-decadal period of drying summertime conditions. Intrinsic water-use efficiency significantly increased for both populations during the past 150 years, but there were no significant differences between the visually healthy and MPB-infected chronologies.
Keywords: Basal area increment, growth divergence, intrinsic water-use efficiency, Montana, mountain pine beetle outbreak, Ponderosa pine, USA
Introduction
The ongoing mountain pine beetle (MPB; Dendroctonus ponderosae Hopkins) epidemic affecting pine species in western North America is the largest in recorded history (Mitton and Ferrenberg 2012). During 2001–2010, over 2.3 million hectares of forests in Montana were affected by the MPB (Chaney 2011), which caused mortality to numerous species of the Pinus genus including lodgepole (P. contorta), ponderosa (P. ponderosa), and whitebark (P. albacaulis) pines (Logan et al. 2010). Mortality rates peaked in 2009 when approximately 1.5 million hectares of pines experienced MPB-caused death. The MPB is native to western North America, with occasional years marked by outbreak populations (Brunelle et al. 2008) triggered by favorable periodic climatic and/or stand conditions (Gibson et al. 2009), but typically only low levels of infestation and subsequent tree mortality occur in a given year.
The magnitude of the current epidemic has been attributed to multi-decadal climate change (Logan and Powell 2001; Akuma et al. 2008; Bentz et al. 2010) directly through a reduction in low temperature-induced mortality caused by extended cold-air incursions, particularly during the autumn and spring, and indirectly through increased drought frequency. Carbon assimilation is impacted by reduced stomatal conductance and reduced photosynthate allocations for defense mechanisms or tissue repair during drought periods, making the infected trees more susceptible to mortality. Additionally, warmer conditions have allowed a change from univoltine to bivoltine life cycles (one to two generations) in some broods of MPB (Mitton and Ferrenberg 2012). Extensive fire-suppression activities that decrease the number and extent of stand-replacing fires can also affect forest susceptibility to MPB outbreaks (Kulakowski et al. 2012).
During the summer months, MPB attack trees en-mass and girdle the phloem that in turn decreases photosynthate transport within a tree and reduces tree health (Gibson et al. 2009). MPB also carry the spores of two “blue stain” fungal species, Ophiostoma montium and Grosmannia clavigera, that are inoculated into the trees by adult beetles. The fungi subsequently spread in the sapwood and phloem of infected trees, reducing water transport and altering resin flow in a fashion that contributes to an increase in tree mortality (Gibson et al. 2009). Mass-attacked trees typically die the following growing season, although trees subject to partial or “strip” attacks may survive several years post infestation (Gibson et al. 2009).
Despite the widespread mortality in forest stands, not all trees are affected, and the reasons for variations in host susceptibility remain uncertain. Tree vigor, a measure of wood production/leaf area, is significantly inversely correlated with individual tree susceptibility (Larsson et al. 1983; Mitchell et al. 1983) and is influenced by stand density, pathogenic agents, and fluctuations in precipitation and temperature (Gibson et al. 2009). Ponderosa pine trees are most likely to be susceptible in dense stands (>5.6 m2 basal area/hectare) of adult tress (>80 years) with diameters of 20–30 cm (Gibson et al. 2009). Tree mortality may increase consistently up to diameters of approximately 23 cm, with mortality of larger trees being more random (McCambridge et al. 1982).
Here, we compare radial growth rates of co-occurring populations of mature healthy and MPB-infected ponderosa pine trees collected at two sites in western Montana. Our objectives were to: (1) compare basal area increment values within populations and between sites; (2) examine the potential influence of changes in intrinsic water-use efficiency; (3) compare climate-growth responses; and (4) postulate on what increases individual tree susceptibility to MPB-induced mortality within a stand where other growth-influencing factors are held constant.
Methods
Tree-ring data collection
We collected samples in western Montana at Cabin Gulch Research Natural Area 28 km NE of Helena and Kitchen Gulch 32 km SE of Missoula (Table 1). The two sites were selected because they had a history of minimal anthropogenic impacts including livestock grazing, wood cutting, and fire suppression (pers. comm., Steve Shelly, USFS R1 RNA Coordinator) and contained both visually healthy and MPB-infected trees of multiple age classes growing in park-like stands (Fig. 1). At each site, we sampled visually healthy and MPB-infected trees using standard dendroecological field techniques, selecting older trees (>150 years) at each site using visual old-age characteristics. Only open-grown trees (Fig. 1) were cored to ensure that radial growth was not affected by canopy closure during, at least, the past century. Both sites are located on steep (25–40%), south-facing slopes with thin soils ranging from 20 to 50 cm (authors' observations).
Table 1.
Site characteristics for Cabin Gulch and Kitchen Gulch
| Site Name | Latitude (°N) | Longitude (°W) | Elevation (m) | Climate division | DBH- visually healthy (cm) | DBH-infected (cm) |
|---|---|---|---|---|---|---|
| Cabin Gulch | 47.79 | 111.77 | 1140 | Montana 4 | 32.44 | 27.53 |
| Kitchen Gulch | 46.71 | 113.65 | 1444 | Montana 1 | 34.4 | 35.95 |
Figure 1.

Visually healthy and MPB-infected trees at Kitchen Gulch, Montana. Photograph by authors, August 2011.
We identified visually healthy trees by the presence of green needles and no evidence of pitch tubes, boring dust, and live MPB eggs, pupae, larvae, or adults. MPB-infected trees were selected by the presence of red foliage in the crown and upon coring, blue-stained sapwood caused by the blue stain fungi. We additionally examined beetle gallery patterns on selected trees to confirm MPB activity opposed to other boring beetles. For each tree, we collected two core samples using increment borers at approximately 1.4-m height. Additionally, we measured crown height and basal diameter for each tree.
We processed collected cores using standard dendrochronology procedures (Stokes and Smiley 1968). We cross-dated the cores using the list method (Yamaguchi 1991), and confirmed dating accuracy using the diagnostic program COFECHA (Holmes 1983). We used raw ring-width data to calculate basal area increment (BAI, cm2 yr−1) for all cores from 1860 to 2007/8 for each of the four chronologies (CGR [visually healthy and developed from 12 trees and 20 cores] and CGI [MPB-infected; 10 trees, 18 cores] for Cabin Gulch and KGR (21 trees, 39 cores), and KGI (16 trees, 30 cores) for Kitchen Gulch). To account for the potential influences of variations in tree size and age between chronologies from the same site, we correlated unstandardized BAI values with standardized BAI values for each chronology. We found negligible differences between the chronologies (all r-values > 0.97) and then retained unstandardized BAI values for our remaining analyses as this allowed for direct comparisons of BAI values within and between our study sites.
Carbon isotope analysis
We developed carbon isotope chronologies from both the visually healthy and MPB-infected trees at Cabin Gulch following methods discussed in Knapp and Soulé (2011). For each chronology, we selected six trees based on the longest and clearest ring structure, with two samples representing each tree. We manually separated tree rings for each sample into pentads (5-year segments) beginning in AD 1800 or later (because of lack of older cores) using a scalpel. We pooled the pentads from all samples and ground together to 40 mesh. To quantitate inter-tree isotopic variability, we also pooled samples for each 50-year period. We processed all ground samples to α-cellulose following conversion to holocellulose using the Jayme-Wise method (see Leavitt and Danzer 1993) and using batch processing and commercial digestion pouches (ANKOM Technology, Boston, MA). Sample extractions were first removed using a soxhlet extraction apparatus operating with toluene/ethanol and ethanol organic solvents followed by boiling in deionized (DI) water. Lignin was removed by reaction in an acetic acid-acidified, sodium chlorite aqueous solution at 70°C and then rinsed thoroughly in DI water. Final isolation of α-cellulose was achieved by treatment in 17% NaOH solution following methodology identified in Sternberg (1989) with samples being rinsed in DI water and dried at 70°C.
The α-cellulose was combusted to CO2 in a Thermo-Finnigan TC/EA and then delivered to a Thermo Finnigan Delta Plus XL mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA) operating in a flow-through mode with a Conflo III interface. We determined isotopic composition with respect to the PDB standard (Coplen 1996). For every four to five samples, a homogenous acetanilide working standard of known isotopic composition was run for mass spectrometer calibration to insure that machine precision of ca. 0.02–0.11‰ (SD) was present for each batch of 30–40 samples analyzed at the University of Arizona, Laboratory of Tree-Ring Research. Additionally, we used a holocellulose laboratory standard (MAWS) for approximately every 15 samples, which established precision of 0.12‰.
We determined intrinsic water-use efficiency (iWUE) following the equation of Ehleringer and Cerling (1995):
| (1) |
where A = CO2 assimilation of ponderosa pine leaves, g = leaf stomatal conductance, 0.625 = constant, and ci/ca = intercellular CO2 and atmospheric CO2. We interpolated annual ca with decadal observations beginning in 1800 (Etheridge et al. 1998) and with measurements collected at Mauna Loa from 1959 to 2009 (Keeling et al. 2008). Equations 2 (carbon isotopic discrimination for plants Δ) and 3 (the ci/ca ratio; Farquhar et al. 1982) allowed us to obtain (ci):
| (2) |
| (3) |
We derived the stable carbon isotopic composition of the atmosphere (δ13Ca) from 1796 to 2002 using data from Francey et al. (1999) and Allison et al. (2003). Because yearly data were temporally incomplete until 1991, we used a sixth-order polynomial to create annual resolution data (e.g., Hemming et al. 1998). Holding all pre-1850 data stable at -6.4‰, we averaged δ13Ca values by pentad. We also averaged isotopic composition of the trees that were pooled by site (δ13Cp) by pentad. Respectively, a and b represented constants for the discrimination during diffusion of CO2 into the air (4.4‰) and discrimination during carboxylation (assumed at 27‰).
Statistical analysis
We used Spearman correlation (PASW Statistics 18.0) to identify the principal climatic drivers of radial growth for each site paired with data from climatic divisions (NCDC 1994) Montana-1 (MT1, Kitchen Gulch) and Montana-4 (MT4, Cabin Gulch) during 1895–2007/8. We examined monthly and seasonal precipitation, temperature, and drought severity influences, including lagged influences up to 1 year.
We examined potential possibilities for increased MPB susceptibility by comparing BAI values between visually healthy and MPB-infected populations at each site during stress and non-stress years using the non-parametric Mann–Whitney U-test. During 1895–2007/8, we categorized all years with Palmer Drought Severity Index (PDSI) values <−2.0 (moderate drought or greater) as stressful, or PDSI values > −0.5 (normal to wet) as non-stressful. We identified potential shifts in iWUE at Cabin Gulch following the methodology of Rodionov (2004) and Rodionov and Overland (2005). We used a significance level of P < 0.05 and cut-off length of 30 years to detect possible multi-decadal-length changes in iWUE since 1860.
Results
Climate/growth relationships
Climate–growth relationships indicated that the principal climate driver of growth was significantly correlated (P < 0.05) with July PDSI for each chronology. At Cabin Gulch, there was a large difference between chronologies (rs = 0.42 CGR, n = 20 cores and rs = 0.62 CGI, n = 18), but less difference existed between chronologies at Kitchen Gulch (rs = 0.55 KGR, n = 39; rs = 0.53 KGI, n = 30). During 1895–2008, July PDSI for MT4 had a downward linear trend (rs = −0.34, P < 0.01) while July PDSI for MT1 exhibited no significant change (rs = −0.11 P = 0.24), indicating increasingly drier summer conditions at Cabin Gulch and stable conditions at Kitchen Gulch. During the last decade (1999–2008), however, there was severe summer dryness at Cabin Gulch, with all years having negative PDSI values and a mean PDSI value of −2.61. Kitchen Gulch also experienced dry July PDSI conditions over the same period  , but 2 years had PSDI values > 0.
, but 2 years had PSDI values > 0.
Basal area increment
Basal area increment for MPB-infected and visually healthy trees at each site closely matched during the majority of the 150-year period (Figs. 2, 3), but diverged during the last two–three decades. Examining differences using 30-year intervals, mean BAI for visually healthy trees at Cabin Gulch was significantly less during 1890–1919 and significantly greater during 1980–2007 when compared with the MPB-infected sample (Table 2). At Kitchen Gulch, mean BAI was significantly greater during 1980–2008 when compared with the MPB-infected sample (Table 2) and for the average of two visually healthy chronologies compared with the average of the MPB-infected samples. The inflection point for radial growth divergence occurred ca. 1980 for Cabin Gulch, with the visually healthy trees maintaining higher BAI values for every year and the largest differences beginning in the mid-1990s (Fig. 2). A similar but less pronounced pattern occurred at Kitchen Gulch, with consistently higher BAI values for the visually healthy trees beginning in the early 1990s and the largest differences occurring during the last decade (Fig. 3). The combined chronologies showed no differences between infected and healthy trees until 1980–2007.
Figure 2.

BAI values (cm2 yr−1) for the two chronologies developed at Cabin Gulch during 1860–2007.
Figure 3.
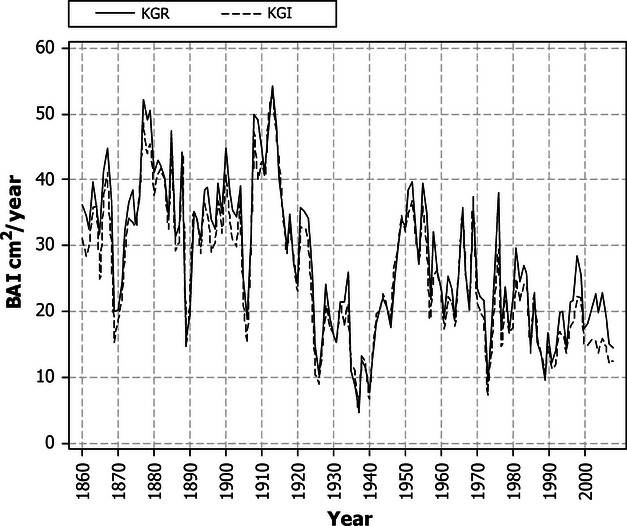
BAI values (cm2 yr−1) for the two chronologies developed at Kitchen Gulch during 1860–2008.
Table 2.
BAI comparison between chronologies for MPB-infected and visually healthy trees at both sites and the two sites combined for 30-year periods (AD 1860–2007/8). Shaded boxes indicate significance at p < 0.05
| Period (AD) | Chronology | Segment length (Years) | Mean BAI | Std. deviation (BAI) | Independent samples M–W U-test (one-tailed) |
|---|---|---|---|---|---|
| 1860–1889 | KGR | 30 | 36.60 | 8.77 | |
| KGI | 30 | 33.76 | 8.80 | 0.092 | |
| 1890–1919 | KGR | 30 | 36.84 | 8.44 | |
| KGI | 30 | 34.81 | 8.59 | 0.145 | |
| 1920–1949 | KGR | 30 | 19.83 | 8.41 | |
| KGI | 30 | 19.06 | 7.92 | 0.351 | |
| 1950–1979 | KGR | 30 | 26.33 | 7.90 | |
| KGI | 30 | 24.42 | 7.63 | 0.159 | |
| 1980–2008 | KGR | 29 | 19.37 | 5.09 | |
| KGI | 29 | 16.62 | 4.12 | 0.025 | |
| 1860–1889 | CGR | 30 | 11.08 | 2.89 | |
| CGI | 30 | 12.51 | 3.81 | 0.07 | |
| 1890–1919 | CGR | 30 | 9.43 | 3.18 | |
| CGI | 30 | 11.59 | 4.47 | 0.02 | |
| 1920–1949 | CGR | 30 | 8.86 | 2.03 | |
| CGI | 30 | 9.36 | 2.50 | 0.21 | |
| 1950–1979 | CGR | 30 | 10.24 | 2.29 | |
| CGI | 30 | 9.37 | 2.59 | 0.12 | |
| 1980–2007 | CGR | 28 | 10.45 | 2.87 | |
| CGI | 28 | 7.82 | 2.67 | 0.001 |
| Sites combined | |||||
|---|---|---|---|---|---|
| 1860–1889 | Visually healthy | 30 | 23.82 | 5.55 | |
| Infected | 30 | 23.14 | 5.89 | 0.282 | |
| 1890–1919 | Visually healthy | 30 | 23.13 | 5.42 | |
| Infected | 30 | 23.20 | 6.14 | 0.477 | |
| 1920–1949 | Visually healthy | 30 | 14.34 | 4.71 | |
| Infected | 30 | 14.21 | 4.59 | 0.474 | |
| 1950–1979 | Visually healthy | 30 | 18.28 | 4.69 | |
| Infected | 30 | 16.90 | 4.63 | 0.131 | |
| 1980–2007 | Visually healthy | 28 | 15.06 | 3.46 | |
| Infected | 28 | 12.29 | 2.97 | 0.002 |
Mean BAI values at Cabin Gulch were 18% higher (n = 27 years; P < 0.01, one-tailed) for the visually healthy trees compared with MPB-infected trees during stressful years (PDSI<−2.0), but the two populations were not different (n = 66; P = 0.781, two-tailed) during non-drought years (PDSI > −0.5). At Kitchen Gulch, BAI values of healthy trees were not significantly greater during either drought (n = 26 years, P = 0.19, one-tailed) or non-drought years (n = 69; P = 0.17, two-tailed).
Intrinsic water-use efficiency
Intrinsic water-use efficiency at Cabin Gulch was not significantly different (P > 0.05) for visually healthy and infected samples during 1860–2009 (101.5 μmol/mol vs. 100.9 μmol/mol) and 1980–2009 (118.3 vs. 117.7). Both samples experienced upward trends in iWUE, with significant (P < 0.05, 30-year cut-off) shifts toward higher iWUE beginning in 1955–59 for the visually healthy trees and 1960–1964 for the infected trees (Fig. 4). Mean iWUE between the early and shift points was 96.3 vs. 113.75 (18% increase) and 96.2 vs. 110 (17% increase) for the visually healthy and the MPB-infected populations.
Figure 4.
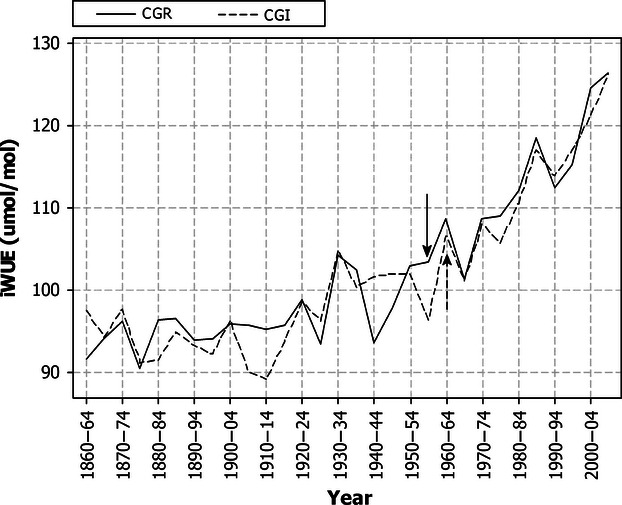
Intrinsic water-use efficiency (μmol/mol) for visually healthy (CGR) and MPB-infected trees (CGI) at Cabin Gulch (pentads from AD 1860–2009). Vertical arrows indicate regime shift (p < 0.05) inflection points.
Discussion
Radial growth divergence
Basal area increment values within populations and between sites were similar until the late 20th Century, at which point the MPB-infected populations had consistently lower BAI values. Thus, growth rates 20–30 years prior to the current outbreak diverged between our selected populations with the slower growing trees being more vulnerable to infestation. These findings are consistent with the vigor hypothesis (e.g., Larsson et al. 1983), and the findings may be revealing in that these were open-grown trees sampled from the same slope, soils, and aspect as visually healthy trees. Sampled trees from the chronologies were often (approximately 50% of total) collected within 15 m of each other, further reducing the likelihood that growth differences were an artifact of microsite variability.
Although changes to higher iWUE occurred slightly earlier for the visually healthy chronology, iWUE trended upward for both chronologies at Cabin Gulch with only minor and non-significant differences between populations. Thus, changes in iWUE do not explain the growth divergences. Growth/climate responses also were similar, with both populations at both sites dependent on summer soil-moisture conditions as inferred from July PDSI values. At Cabin Gulch, the infected population exhibited greater growth sensitivity to climate, which is consistent with the findings of McDowell et al. 2010, who found greater drought-induced mortality in ponderosa pine with increasing climatic sensitivity. Conversely, at Kitchen Gulch, the healthy population exhibited slightly greater sensitivity to climatic variations than the infected population, suggesting that the relationship between vigor and climatic sensitivity may be site specific.
Intrinsic water-use efficiency
Our findings also suggest that significant increases in iWUE do not confer immunity to MPB infestations for all trees during moisture-stress periods, but may have the potential to lessen the severity of the attacks because of the ameliorative effects of improved plant–water relationships. In the Northern Rockies, USA, radial growth rates and iWUE of ponderosa pine are significantly and positively associated with age, suggesting that the water stress-ameliorating effects of increased atmospheric CO2 preferentially favor older trees (Knapp and Soulé 2011). In this study, median tree age at CGR (260 years) and KGR (180 years) were greater than for CGI (207 years) and KGI (156 years). Our findings are consistent in that older trees may have been less affected by drought conditions than younger trees during the last 30 years, and in turn have been less susceptible to MPB infestations due to the reduction in moisture stress, particularly at Cabin Gulch where age differences (53 years) were more pronounced.
Implications
The divergence in growth shown between populations at these sites may reveal “previously invisible genetic quirks” (Neese and Williams 1994:144) that may favor/disfavor different genotypes embedded within a local population when stress occurs. For both sites sampled, MPB-infected and visually healthy populations followed two different growth trajectories in the late 20th Century/early 21st Century, suggesting that some type of environmental shift favored one subgroup of ponderosa pine over the other prior to the onset of MPB attacks. In turn, the less-vigorous trees were unable to repel MPB attacks, making them more vulnerable to the current epidemic. For the Cabin Gulch and Kitchen Gulch chronologies, the visually healthy trees were less affected by drought conditions as they maintained higher BAI values during the past 20–30 years, although this relationship was better expressed at Cabin Gulch where the overall climatic conditions are drier than at Kitchen Gulch and age differences between groups were less. Conversely, the lack of pronounced superior growth of the healthy trees throughout the record suggests other possibilities for divergence. For example, prior MPB infestations that weakened, but did not kill, the trees 20–30 years ago may have made the MPB trees more susceptible to the current episode.
Overall these results were similar between the Cabin Gulch and Kitchen Gulch sites. Radial growth divergence occurred several decades prior to infection, suggesting that the likelihood of MPB infestation of individual trees older than 150 years is not arbitrary and potentially set in motion decades prior to actual infestations. In short, susceptibility of ponderosa pine to MPB infestations may be strongly associated with the long-term vigor of individual trees that emerges during episodic outbreaks. The consequences of these outbreaks are that they selectively remove the least vigorous trees from the local forest population, thus promoting an overall increase in tree vigor and drought resistance.
Acknowledgments
This research was funded by NSF grant #0851081. We thank Michele Abee, David Austin, and Amanda Roberts for laboratory and field work contributions and Steve Shelly for logistical support and fieldwork assistance. We also thank three anonymous reviewers for their insightful comments and suggestions.
Biographies
Paul Knapp is a professor and director of the Carolina Tree-Ring Science Laboratory in the Department of Geography at the University of North Carolina-Greensboro. His interests have focused on the paleoecological dynamics of western North American forests.
Peter Soule' is a professor in the Department of Geography and Planning at Appalachian State University. His main area of research deals with the aspects of climate and vegetation change in western North America.
Justin Maxwell is an assistant professor in the Department of Geography at Indiana University. His interest areas include climatology, dendrochronology, and forest disturbance in the southeastern and western U.S.
Conflicts of Interest
None declared.
References
- Akuma BH, Carroll AL, Zheng Y, Zhu J, Raffa KF, Moore RD, et al. Movement of outbreak populations of mountain pine beetle: influence of spatiotemporal patterns and climate. Ecography. 2008;31:348–358. [Google Scholar]
- Allison CE, Francey RJ, Krummel PB. δ13C in CO2 from sites in the CSIRO Atmospheric Research GASLAB air sampling network, (April 2003 version). In Trends: A Compendium of Data on Global Change. Oak Ridge, TN, USA: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy; 2003. [Google Scholar]
- Bentz BJ, Régnière J, Fettig CJ, Hansen EM, Hayes JL, Hicke JA, et al. Climate change and bark beetles in the western United States and Canada: direct and indirect effects. Bioscience. 2010;60:602–613. [Google Scholar]
- Brunelle A, Rehfeldt GE, Bentz B, Munson AS. Holocene records of Dendroctonus bark beetles in high elevation pine forests of Idaho and Montana, USA. For. Ecol. Manage. 2008;255:836–846. [Google Scholar]
- Chaney R. Survey shows pine beetle infestation in Montana forests might be declining. Missoulian; 2011. March 29. [Google Scholar]
- Coplen TB. New guidelines for reporting stable hydrogen, carbon and oxygen isotope-ratio data. Geochim. Cosmochim. Acta. 1996;602:3359–3360. [Google Scholar]
- Ehleringer JR, Cerling TE. Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentrations in plants. Tree Phys. 1995;15:105–111. doi: 10.1093/treephys/15.2.105. [DOI] [PubMed] [Google Scholar]
- Etheridge DM, Steele LP, Langenfelds RL, Francey RJ, Barnola J-M, Morgan VI. Historical CO2 records from the Law Dome DE08, DE08-2, and DSS ice cores. In Trends: A Compendium of Data on Global Change. Oak Ridge, Tennessee, USA: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy; 1998. [Google Scholar]
- Farquhar GD, O'Leary MH, Berry JA. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust. J. Plant Phys. 1982;9:121–137. [Google Scholar]
- Francey RJ, Allison CE, Etheridge DM, Trudinger CM, Enting IG, Leuenberger M, et al. A 1000-year precision record of δ13C in atmospheric CO2. Tellus. 1999;51B:170–193. [Google Scholar]
- Gibson K, Kegley S, Bentz B. Mountain pine beetle. USDA; 2009. p. 12. USDA Forest Service Forest Insect & Disease Leaflet 2. [DOI] [PubMed] [Google Scholar]
- Hemming DL, Switsur VR, Waterhouse JS, Heaton THE, Carter AHC. Climate variation and the stable carbon isotope composition of tree ring cellulose: an intercomparison of Quercus robur, Fagus sylvatica and Pinus silvestris. Tellus B. 1998;50:25–33. [Google Scholar]
- Holmes RL. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983;43:69–78. [Google Scholar]
- Keeling RF, Piper SC, Bollenbacher AF, Walker JS. Atmospheric CO2 records from sites in the SIO air sampling network. In Trends: A Compendium of Data on Global Change. Oak Ridge, Tennessee, USA: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy; 2008. [Google Scholar]
- Knapp PA, Soulé PT. Increasing water-use efficiency and age-specific growth responses of old-growth ponderosa pine trees in the Northern Rockies. Glob. Change Biol. 2011;17:631–641. [Google Scholar]
- Kulakowski D, Jarvis D, Veblen TT, Smith J. Stand-replacing fires reduce susceptibility of lodgepole pine to mountain pine beetle outbreaks in Colorado. J. Biogeogr. 2012;39:395–402. [Google Scholar]
- Larsson S, Oren R, Waring RH, Barrett JW. For. Sci. 1983;29:395–402. [Google Scholar]
- Leavitt SW, Danzer SR. Method for batch processing small wood samples to holocellulose for stable-carbon isotope analysis. Anal. Chem. 1993;65:87–89. [Google Scholar]
- Logan JA, Powell JA. Ghost forests, global warming, and the mountain pine beetle (Coleoptera: Scolytidae) Am. Entomol. 2001;47:160–173. [Google Scholar]
- Logan JA, MacFarlane WM, Wilcox L. Whitebark pine vulnerability to climate-driven mountain pine beetle disturbance in the greater Yellowstone Ecosystem. Ecol. Appl. 2010;20:895–902. doi: 10.1890/09-0655.1. [DOI] [PubMed] [Google Scholar]
- McCambridge WF, Hawksworth FG, Edminster CB, Laut JG. Ponderosa pine mortality resulting from a mountain pine outbreak. USDA: USDA Forest Service Research Paper RM235; 1982. p. 7. [Google Scholar]
- McDowell NG, Allen CD, Marshall L. Growth, carbon-isotopic discrimination, and drought-associated mortality across a Pinus ponderosa elevational transect. Glob. Change Biol. 2010;16:399–415. [Google Scholar]
- Mitchell RG, Waring RH, Pitman GB. Thinning lodge pine increases tree vigor and resistance to mountain pine beetle. For. Sci. 1983;29:204–211. [Google Scholar]
- Mitton JB, Ferrenberg SM. Mountain pine beetle develop an unprecedented summer generation in response to climate warming. Am. Nat. 2012;179:E163–E171. doi: 10.1086/665007. [DOI] [PubMed] [Google Scholar]
- NCDC. 1994. p. 12. Time Bias Corrected Divisional Temperature-Precipitation-Drought Index. Documentation for dataset TD-9640. Available from DBMB, NCDC, NOAA, Federal Building, 37 Battery Park Ave. Asheville, NC 28801-2733.
- Neese RM, Williams GC. Why we get sick. New York: Vintage Books; 1994. p. 273. [Google Scholar]
- Rodionov SN. A sequential algorithm for testing climate regime shifts. Geophys. Res. Lett. 2004;31:L09204. doi: 10.1029/2004GL019448. [Google Scholar]
- Rodionov SN, Overland JE. Application of a sequential regime shift detection method to the Bering Sea ecosystem. ICES J. Mar. Sci. 2005;62:328–332. [Google Scholar]
- Sternberg LDSL. Oxygen and hydrogen isotope ratios in plant cellulose: mechanisms and applications. In: Rundel PW, Ehleringer JR, Nagy KA, editors. Stable isotopes in ecological research. Berlin, Germany: Springer-Verlag; 1989. pp. 124–141. [Google Scholar]
- Stokes MA, Smiley TL. An introduction to tree-ring dating. Chicago: University of Chicago Press; 1968. [Google Scholar]
- Yamaguchi DK. A simple method for cross-dating increment cores from living trees. Can. J. For. Res. 1991;21:414–416. [Google Scholar]


