Table 1.
Optimization of the Pd-catalyzed ortho-C–H arylation of benzylpicolinamide. All screening reactions were carried out in a 10 mL glass vial on a 0.2 mmol scale.
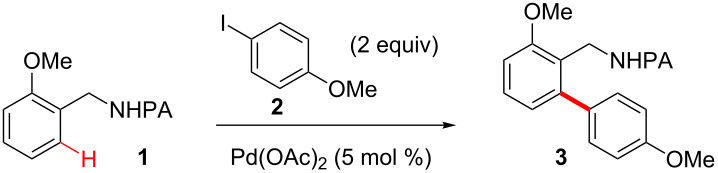 | ||||
| entry | additives (equiv) | temperature (°C) | solvent | yield of 3 (%)a |
| 1 | AgOAc (1.5) | 150 | no solvent | 76 |
| 2 | AgOAc (1.5) | 120 | toluene | 6 |
| 3 | AgOAc (1.5), PivOH (0.3) | 120 | toluene | 3 |
| 4 | PivOH (0.3) | 120 | toluene | 5 |
| 5 | K2CO3 (2) | 120 | toluene | 57 |
| 6 | PivOH (0.3), K2CO3 (2) | 120 | toluene | 90 |
| 7 | PivOH (0.3), KHCO3 (2) | 120 | toluene | 95 (91)b |
| 8 | AcOH (0.3), KHCO3 (2) | 120 | toluene | 78 |
| 9 | oPBAc (0.3), KHCO3 (2) | 120 | toluene | 84 |
| 10 | PivOH (0.3), KHCO3 (2) | 90 | toluene | 29 |
aYields are based on 1H NMR analysis of the reaction mixture after workup; bIsolated yield; coPBA: ortho-phenylbenzoic acid.
