Figure 5. Evidence for oligomerization of hIKK2 dimers in solution.
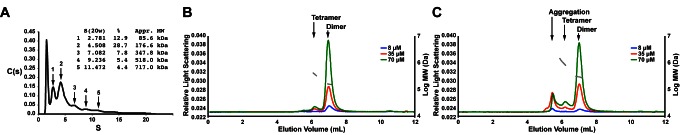
(A) Analytical ultracentrifugation sedimentation velocity experiments on concentrated samples of full-length hIKK2 reveal a pattern that correlates with monomer–dimer equilibrium as well as formation of tetramers, hexamers, and octamers in solution. Arrows mark peaks and a summary of data output including calculated molecular weights is inset. (B) When full-length, ATP-treated hIKK2 is analyzed by SEC-MALLS, one observes peaks that correspond to dimer (major peak) and tetramer (minor peak). (C) The hIKK2 I413A/L414A mutant protein displays defects in its ability to undergo reversible dimer–tetramer transitions without aggregating.
