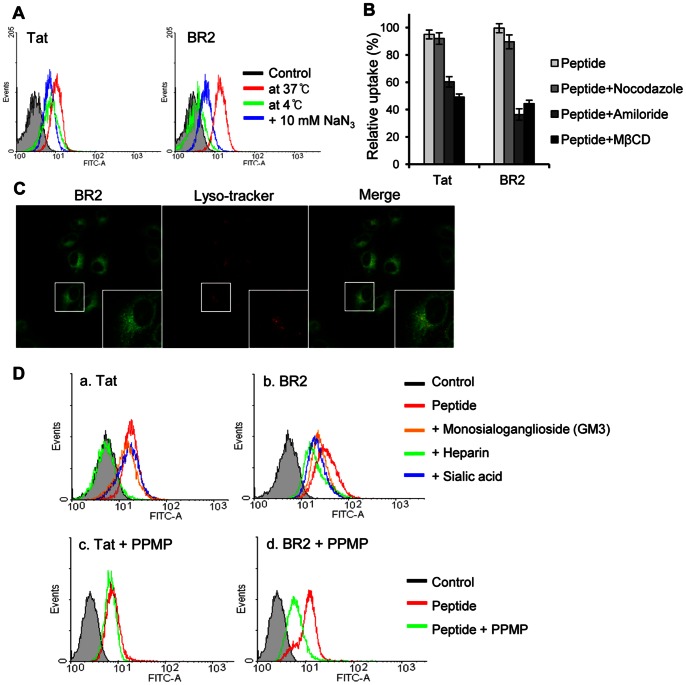
Figure 3. Contribution of energy-dependent pathways and negatively charged molecules on cancer cell membranes to peptide internalization.
(A) Effects of low temperature and energy depletion on the internalization of FITC-labeled peptides into HeLa cells. HeLa cells were either preincubated at 4°C or pretreated with sodium azide (NaN3) to deplete ATP for 1 h, and then incubated with 5 µM Tat or BR2 for 30 min under the same conditions, as described in Materials and Methods. Peptide uptake was determined by flow cytometry. (B) Effects of endocytic inhibitors on the entry of BR2 and Tat. The influence of inhibitory drugs on peptide uptake was determined by preincubation of HeLa cells with the endocytosis inhibitors nocodazole, amiloride or methyl-ß-cyclodextrin for 1 h prior to the addition of FITC-labeled peptides. After peptide treatment for 30 min at 37°C, FITC-positive cells were counted by flow cytometry. Values represent the percentage of fluorescence-positive cells in the total cell population. Data represent the mean ± s.d. of three independent experiments. (C) Colocalization of BR2 with the lysosomal marker LysoTracker red DND-99 in living HeLa cells. After 30 min incubation of BR2 (5 µM) with LysoTracker, live HeLa cell images were obtained by confocal microscopy. (D) Effects of negatively charged molecules (gangliosides, heparins, and sialic acids) on peptide uptake. (a,b) HeLa cells were treated with BR2 and Tat in the presence of gangliosides, heparins, or sialic acids (each, 20 µg/ml) for 30 min. In (c,d), HeLa cells were pretreated with PPMP (5 µM) to deplete gangliosides. Cellular uptake of BR2 and Tat was determined by flow cytometry. All experiments were performed in triplicate.