Fig. 5.
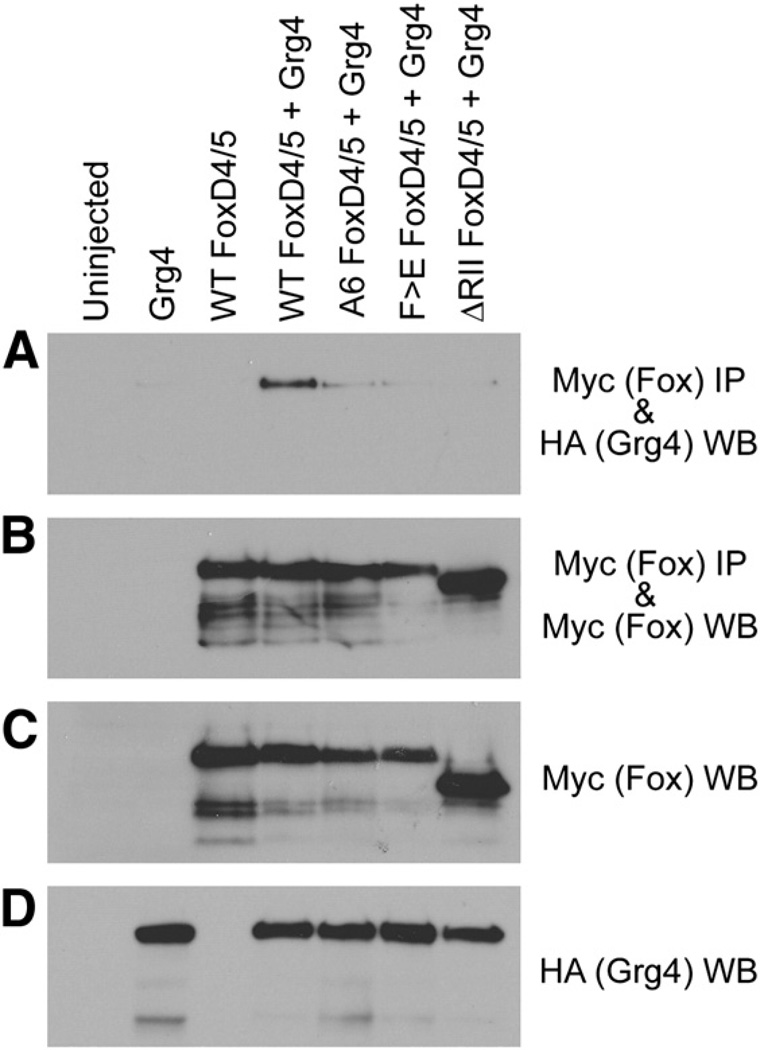
Gro/Grg4 binds to the Eh-1 motif of FoxD4/5. (A–D) Myc-tagged versions of wild-type (WT), as well asmutants harboring amino acid substitutions in the Eh-1 domain (A6, F>E) or deleted for the Eh-1 domain (ΔRII) in FoxD4/5 were expressed in Xenopus oocytes along with HA-tagged wild-type Gro/Grg4 (Grg4). Co-immunoprecipitation (IP) and Western blot (WB) analyses of Xenopus oocyte lysates expressing HA- and Myc-tagged constructs are indicated. (A) Although all constructs are equivalently expressed, only full-length FoxD4/5 effectively binds with Gro/Grg4. The control panels (B–D) show that the IPs each contain similar levels of FoxD4/5 wild-type and mutant proteins (B), as do the direct lysates (C). Gro/Grg4 expressing lysates also show similar levels of this protein (D).