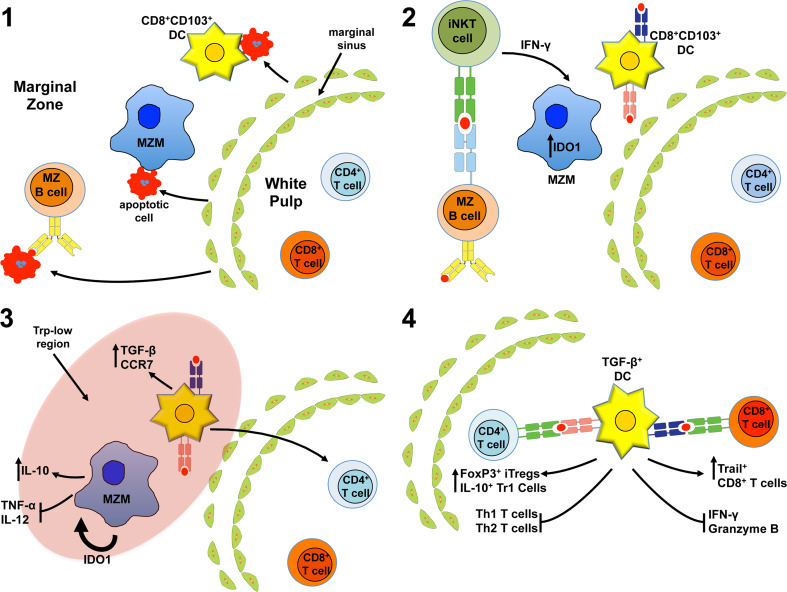
Fig. 2.
Hypothetical model of induced splenic tolerance to apoptotic cells. 1 Circulating apoptotic cells enter the spleen through the marginal sinus traveling towards the red pulp. The apoptotic cells are captured in the marginal zone (MZ), primarily by marginal zone macrophages (MARCO+ MZM) and resident CD8+ DCs. However, MZ B cells also capture apoptotic cells in a B cell receptor-dependent manner. 2 Activated MZ B cells present either endogenous lipids or lipids derived from captured, internalized apoptotic cells in CD1d molecules to invariant NKT cells stimulating rapid production of IFN-γ. IFN-γ stimulates the MZMs to induce intracellular expression of the immunoregulatory enzyme IDO1. 3 IDO1 acts on MZMs in an autocrine and paracrine fashion promoting apoptotic cell-induced IL-10 production while inhibiting proinflammatory cytokine synthesis. Moreover, induction of IDO creates a zone of low tryptophan promoting stress response driven TGF-β production in CD8+ CD103+ tolerogenic DCs. The tolerogenic DCs also up-regulate surface expression of the chemokine receptor CCR7 promoting migration into the PALS. 4 Once in the PALS the TGF-β+CD8+ CD103+ DCs present apoptotic cell antigens by both MHC I and MHC II-dependent mechanisms promoting development of FoxP3+ Tregs, IL-10+ Tr1 T cells (i.e., IL-10+ CD4+ T cells), and Trail+ CD8+ T cells while inhibiting inflammatory CD4+ and CD8+ T cell function, promoting T cell anergy and deletion