Abstract
This study investigates the impact of exercises, coffee intakes, and physical trainings on fuel utilization in rats. Ninety-six rats were fed a control diet with either water (C) or coffee (CF; 0.12 g freeze-dried instant coffee/100 g body weight/d). Additionally, the animals go through physical training (TC and TCF) or no training (NTC and NTCF) for 4 weeks. For physical training, animals have to exercise on treadmills for 30 minutes (5 d per week, 15° incline, 0.5-0.8 km/h). At the end of week 4, the animals in each group were subdivided into three exercise groups: before exercise (BE), during exercise (DE), and after exercise (AE). The DE rats exercised on treadmills for 1 hour immediately before being sacrificed. Hemoglobin, hematocrit, glucose, glycogen, protein, triglyceride (TG), and free fatty acid (FFA) levels in the plasma, liver, and skeletal muscle of the rats were compared accordingly. Organ weights were also measured. Coffee-training interaction had a significant impact on heart weight, visceral fat, hemoglobin, hematocrit, liver glycogen in DE and AE, and liver triglyceride in DE and AE. Exercise (meaning exercised on a treadmill for 1 hour immediately before being sacrificed) training interaction was significant in liver glycogen, muscle glycogen in control diet and control diet with coffee, FFA and muscle TG levels at control diet with coffee group. Exercise-coffee interactions significantly influenced the FFA with no training groups. Exercise-coffee-training interaction significantly effects on FFA, Liver TG and Muscle TG. Coffee intakes can increase lipolysis during exercising but coffee consumptions delay the recovery of liver glycogen levels in trained rats after exercising. Coffee intakes can increase lipolysis during exercising but coffee consumptions delay the recovery of liver glycogen levels in trained rats after exercising. Coffee can be an effective ergogenic aid during exercise for physically trained rats.
Keywords: Coffee, training, exercise, glycogen, free fatty acid
Introduction
Many factors which affect the fuel utilization in the body have been reported and most of the study has been done on each factor separately. However, there are limits when applying the results of these studies to real life since most of the athletes are adjusted to many factors which may affect the fuel utilization simultaneously in order to improve their performance. Athletes have searched for dietary supplements which can enhance their exercise performances. Some nutrients or dietary supplements improve the exercise capability by delaying the onset of fatigue [1]. For example, enhancing lipid oxidation and slowing the rates of muscle glycogen use [2] are very important because depletion of muscle and liver glycogen stores can become a limiting factor during prolonged exercising, and a large amount of energy can be obtained from fat utilization through certain metabolic processes [3]. Physical training facilitates the mobilization and oxidation of fat, as mitochondria increases, it enhances more fatty acid oxidation during exercising, and thus helps to conserve limited carbohydrate storage [4]. Utilization of fuel sources during exercise not only depends on the fuel availability but also on the physical training. When the body undergoes physical training, certain metabolic processes occur to assure that adequate energy is provided to the active muscles [5,6].
The potential effects of several foods or beverages on muscle lipid utilization during exercise are also being investigated [7,8]. Coffee is a rich source of bioactive phytochemicals which includes methyl-xanthines, amino acids, phenolic acids, and polyphenols. Caffeine, the primary methylxantine in coffee, is widely known for its stimulatory and metabolic effects [9,10] which enhances lipolysis and fat oxidation, and reduces glycogen breakdown [11,12]. Phenolic and polyphenolic compounds in coffee increase fatty acid oxidation and insulin sensitivity [13,14], and modulate glucose absorption and utilization [15-17]. Thus, the purpose of this study is to investigate the interaction of the major factors which is being reported to affect the fuel utilization and to be able to apply the results of study to real life.
Materials and Methods
Experimental diets
Ninety-six 4-week old male, Sprague-Dawley rats (Daehanbiolink Co., South Korea) weighing 95-105 g were fed a control diet with either water (C) or coffee (CF). The control diet was a vitamin-free, casein-based, semi-synthetic diet which met the AIN-93 recommendations (the American Institute of Nutrition with the objective of standardizing studies in experimental nutrition). In this study, coffee was processed as a solution made from freeze-dried instant coffee (Dongseo Co., South Korea). The average amount of coffee intakes per rat was 0.12 g freeze-dried instant coffee/100 g body weight (BW)/d. This concentration is based on the following rationale. To achieve the visible effects of coffee intake, we maximized the amount of coffee processed based on the approximate maximized quantity reportedly consumed by physically active individuals (about 895 mg of caffeine/60 kg/d) [18]. Additionally, previous studies reported the caffeine contents of different food sources including 106 mg × [1 serving] in espresso, 58 mg × [100 mL] in coffee, 25 mg × [1,000 mL] in black tea, and 15 mg × [100 mL] in green tea [19,20]. Thus, based on the estimation that 150 mL of coffee contains 87 mg of caffeine, 10 cups of coffee (7.2 g of freeze-dried instant coffee per cup) contains 870 mg of caffeine, this would not exceed the upper limit of daily caffeine consumptions for a 60-kg human. If an analogous amount of caffeine were administered to rats accordingly to weight, the animals would be given 0.12 g of coffee/100 g BW/d, which is equivalent to 1.45 mg of caffeine/100 g/d. The study protocol was approved by the Committee on Animal Welfare Regulations of Duksung Women's University, Seoul 132-714, South Korea.
Exercise regime and sample collection
The rats were fed the control diet with either water (C; 48 rats) or coffee (CF; 48 rats). Half of the animals from both groups (24 rats per group) underwent physical training (TC and TCF) or remained stationary (NTC and NTCF) for 4 week. For physical training, the rats exercised on a treadmill (30 min/d, 15° incline, 0.5-0.8 km/h) 5 days per week, and received electric shocks if they did not keep up with running on the treadmills. At the end of week 4, the animals in each dietary groups were subdivided into three groups (eight rats per group) based on exercises: before exercise (BE), during exercise (DE), and after exercise (AE). The BE groups were sacrificed without having performed any exercising at the end of week 4. The DE groups exercised on a treadmill (15° incline, 0.5-0.8 km/h) for 1 hour, and animals in the AE groups were allowed to rest for 1 hour after exercising and then both of groups were sacrificed. At the indicate time intervals, the animals were sacrificed by decapitation while the others were put out of conscience. Immediately after decapitation, blood samples were collected in heparinized tubes (BD Vacutainer®). All blood samples were immediately centrifuged (1300 RCF for 20 min at 4℃) to isolate plasma and erythrocytes. Heart, kidney, liver, and skeletal muscle from the medial red gastrocnemius were rapidly removed and stored at -70℃ until further analysis.
Biochemical analysis
Glycogen was measured by using a colorimetric procedure as previously described [21]. After tissue samples were homogenized (Omni THQ Digital Tissue Homogenizer) in cold sodium phosphate buffers (2 mL, 0.02 M, pH 7.0), aliquots of the homogenates were analyzed for protein and triglyceride contents. Total protein concentrations were determined using a commercial kit (Asan Pharmaceutucal Co., South Korea) based on the Biuret reaction [22]. Triglycerides were analyzed with a commercial kit (Asan Pharmaceutical Co.) utilizing the glycerol phosphate oxidase-quinoneimine colormetric method [23]. Plasma glucose levels were determined with a commercial kit (Youndong Pharmaceutical Co., South Korea) based on an enzymatic method [24]. Free fatty acid (FFA) levels were measured with a commercial kit (NEFAZYME-S, Eiken Chemical Co., Japan) utilizing acyl CoA synthetase-Acyl CoA oxidase [25].
Statistical analysis
Data were analyzed according to a two-way analysis of variance (ANOVA) with coffee intakes and trainings as variables, and a three-way ANOVA with exercises, trainings, and coffee intakes as variables. When significant interactions were identified, Scheffe's post hoc tests were performed. When a significant effect of exercise, coffee, or training being the main factor was observed and the interaction was not significant, a one-way ANOVA was used to compare the means of the main factor (between BE, DE, AE or control diet, control diet with coffee or training, no training). All analysis had been demonstrated with SAS 9.1 for Windows (SAS Institute, Inc., Cary, NC) where P-values < 0.05 were considered statistically significant.
Results
Organ weights and visceral fat
Table 1 shows organ weights for the twelve groups. Training had a significant effect on the heart (P = 0.0436) and visceral fat (P < 0.0001) weight. Since the interactions between coffee intakes and physical trainings were absent, the independent effects of training can be analyzed according to heart weight. The heart weights were significantly higher in the two training groups (TC, TCF) than the two control groups (NTC, NTCF). The combined visceral fat masses were significantly lower in the two training groups (TC, TCF) than the two non-training groups (NTC, NTCF). No significant effects on spleen and liver weights were being observed.
Table 1.
The effects of physical training and coffee intake on the weight of various organs and visceral fat
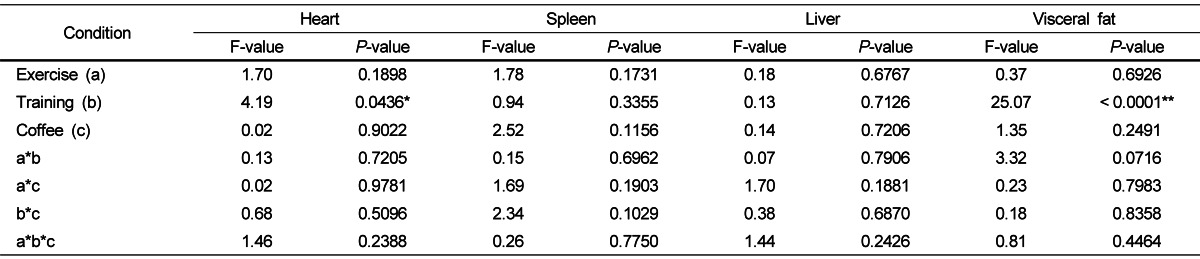
F- and P-values were determined by a three-way ANOVA. * P < 0.05, ** P < 0.01.
Hemoglobin and hematocrit variables
Table 2 shows the hemoglobin and hematocrit variables of the four groups. Training, coffee, and a combination of coffee and training had a significant impact on hemoglobin (P < 0.0001, 0.0001, and 0.0068, respectively) and hematocrit (P < 0.0001, 0.0436, and 0.0008, respectively) variables. The TCF group had lower hemoglobin levels than other three groups while the NTCF group showed lower hematocrit levels than the other three groups.
Table 2.
The effects of physical training and coffee intake on hemoglobin and hematocrit levels
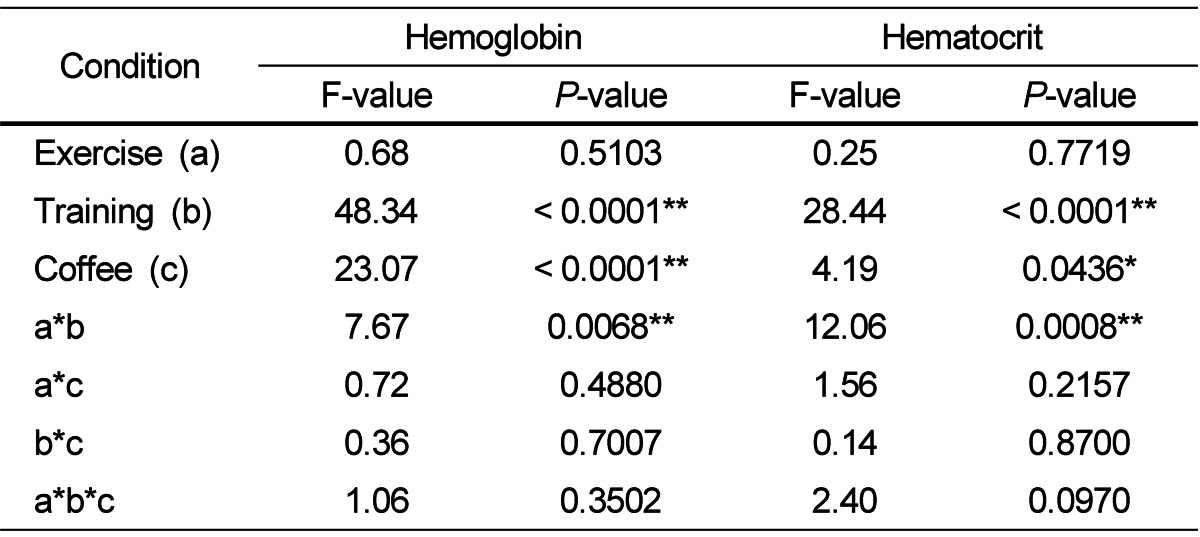
F- and P-values were determined by a three-way ANOVA. * P < 0.05, ** P < 0.01.
Carbohydrate storage variables
Glucose, liver glycogen, and muscle glycogen variables in the four groups (NTC, NTCF, TC, TCF) under exercise (BE, DE, AE) are shown in Table 3. Exercises, trainings, coffee intakes, or any other interactions were found to be insignificant to affecting glucose levels. Training alone or combinations of exercise and training or coffee- and training had significant effects on liver glycogen (P < 0.0001, 0.0175, and 0.0136, respectively). The TCF-BE group had the highest liver glycogen levels while the NTCF-DE group had the lowest out of the TCF-DE, TCF-AE, NTCF-BE, NTCF-AE groups. Coffee intake decreased liver glycogen levels in the T group, but no significant differences were observed in the NT group at DE or AE. Exercise and a combination of exercises and trainings significantly affected muscle glycogen levels (P = 0.0153 and 0.0071, respectively). TC-BE showed significantly higher muscle glycogen levels than NTC-DE at control groups. TCF-BE showed significantly higher muscle glycogen levels than NTCF-DE and NTCF-AE at coffee intake groups.
Table 3.
Effects of exercise, physical training, and coffee intake on the concentrations of glucose, liver glycogen, and muscle glycogen
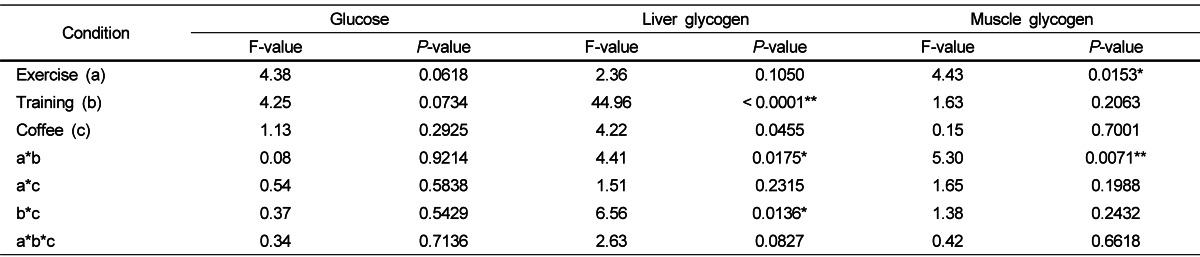
F- and P-values were determined by a three-way ANOVA. * P < 0.05, ** P < 0.01.
Lipid storage variables
Table 4 shows FFA, liver TG, muscle TG, and visceral fat variables in the four groups (NTC, NTCF, TC,TCF) under exercising (BE, DE, AE). Exercises, trainings, and coffees as well as exercise-training, exercise-coffee, and exercise-training-coffee combinations had significant impacts on FFA levels (P < 0.0001, 0.0079, < 0.0001, < 0.0001, 0.0350, and < 0.0001, respectively). A combination of exercise-training significantly affected the controlled diet with coffee solution group. The TCF-DE group showed the highest FFA level while the TCF-BE group had lower FFA levels than NTCF-BE, NTCF-DE, NTCF-AE, TCF-AE. The interaction of exercise and coffee was significant for the no training group. NTCF-BE, NTCF-DE, and NTC-DE rats had significantly higher FFA levels than the NTC-BE, NTC-AE, and NTCF-AE groups. The TCF-DE group had significantly higher levels of FFA while TC-BE, NTCF-AE, and TCF-BE groups had significantly lower levels of FFA than other experimental groups. Exercises, trainings, and coffees alone as well as combinations of training-coffee and exercise-training-coffee significantly affected liver TG concentrations (P = 0.0001, 0.0295, 0.0007, 0.0170, and 0.0060, respectively). The trainingcoffee combination had a significant impact in the DE and AE groups. TC animals showed higher TG levels than the TCF group at DE. Otherwise, the NTC group had significantly higher TG levels than the NTCF group at AE. The NTC-BE and NTC-AE animals had significantly higher liver TG levels while the TCF-DE group had lower liver TG levels than other experimental groups. Muscle TG concentrations were affected by exercising alone, as well as exercise-training and exercise-training-coffee combination (P = 0.0002, 0.0019 and 0.0497 respectively). The effect of the exercise-training combination on muscle TG was significant in the coffee intakes group. Compared to other experimental groups, the TCF-BE group showed significantly higher muscle TG levels while these were significantly lower in the TCF-AE group. CNT-BE rats had significantly higher TG than other groups, CFT-AE groups had significantly lower levels of muscle TG levels than other experimental groups.
Table 4.
Effects of exercise, physical training, and coffee intake on the concentrations of plasma free fatty acids (FFA), liver triglyceride (TG), and muscle TG
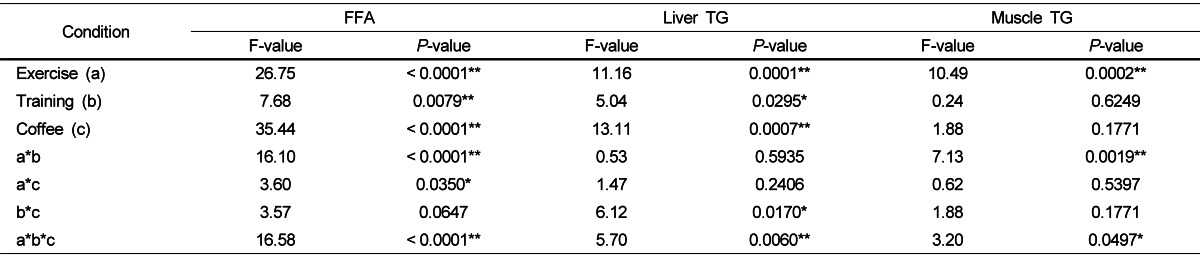
F- and P-values were determined by a three-way ANOVA. * P < 0.05, ** P < 0.01.
Protein storage variables
Table 5 shows the plasma, liver, and muscle protein variables in the four groups (NTC, NTCF, TC, TCF) under exercise (BE, DE, AE). No significant effects on plasma, liver, or muscle protein were observed regardless of exercises.
Table 5.
Effects of exercise, physical training, and coffee intake on the concentrations of plasma, liver, and muscle proteins
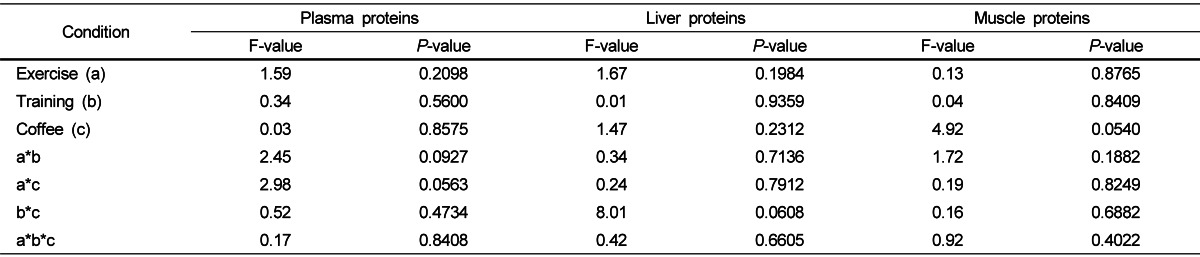
F- and P-values were determined by a three-way ANOVA. * P < 0.05, ** P < 0.01.
Discussion
This study demonstrated that there were significant interactions of coffee intakes, physical trainings and exercises on fuel utilization in vivo in three ways. First, results from the present study indicated that coffee intakes may influence physical training-induced modifications of fuel utilization during exercise. Exercise, training, and coffee intake did not influence blood glucose levels. However, training alone or training-coffee and exercise-training interactions significantly impacted liver glycogen levels. Additionally, training alone or an interaction of exercise and training significantly affected muscle glycogen. Training was the main factor for increased liver and muscle glycogen levels in this study. Training can result in greater glycogen storage that represents an adaptive mechanism which holds greater capacity of trained muscles to maintain their glycogen levels [4]. Liver and muscle glycogen concentrations in the trained groups were significantly higher than those of the animals that were not trained. It has been reported that training increases the sensitivity of muscles to insulin, predominately during the 4 to 6 hours after exercising, and muscle glycogen levels can be restored to near pre-exercise levels within 24 hours. After 24 hours, muscle glycogen concentrations can increase very gradually and eventually surpass normal levels over the next few days [26]. It has also been reported that muscle glycogen synthesis is greater within 2 hours after exercising [27] with the greatest levels observed 45 minutes post-workout.
Coffee could help conserve carbohydrates in the body. The main components of coffee are caffeine, chlorogenic acids, quinidines, and magnesium. Caffeine enhances lipolysis and fat oxidation while reducing glycogen breakdowns [11,12]. Furthermore, caffeine enhances glucose storage through increased release of epinephrine or by antagonizing adenosine receptors [28,29]. Chlorogenic acids and quinidines have antioxidant properties [30] associated with reduced hepatic glucose outputs through the inhibition of glucose-6-phosphatase [15,31], decreased intestinal glucose absorption via the inhibition of glucose-6-phosphate translocase 1 and other mechanisms, and subsequent augmentations of GLP-1 levels [32]. However, an interaction of coffee and training (TCF group) was found to decrease liver glycogen levels in the present study. Additionally, the training group given coffee (TCF) showed delayed recovery of liver glycogen and muscle glycogen levels. These findings suggest that training with coffee intakes may inhibit liver glycogen storage and may delay the recovery of liver and muscle glycogen storages.
Exercise alone can stimulate releasing of epinephrine and raising FFA levels [33]. Caffeine also promotes epinephrine release and the release of fatty acids from adipose tissues, but this does not necessarily mean that fatty acid oxidation is enhanced [34,35]. Exercise, training, and coffee alone along with the exercise-training, exercise-coffee, and exercise-training-coffee interactions significantly affected FFA levels. The TCF-DE group had significantly higher FFA levels while the TCF-BE animals showed significantly lower FFA levels for the coffee intake group. It can be suggested that training decreased plasma FFA levels but coffee intakes enhanced the use of FFAs when exercising. Exercise-coffee interaction was found on FFA for non-trained group. The NTCF group showed higher FFA levels than the NTC animals. Effect of the exercise-training-coffee interaction was illustrated by the observations that the TCF-DE group had significantly higher levels of FFA while these levels were lower for the TC-BE, NTCF-AE, and TCF-BE groups. These findings suggest that training and coffee intake stimulate FFA release in trained rats while exercising.
A significant effect of the coffee-training interaction on liver TG levels at DE and AE was observed. The TC group showed significantly higher liver TG levels than the TCF group at DE. This finding can be explained by the observation that coffee intakes decreased liver TG levels at DE in the trained rats. NTC animals had significantly higher liver TG levels than the NTCF group at AE. Liver is the primary site of FFA synthesis during exercise while glucagon and cortisol stimulate the ß-oxidation of FFAs [36]. It can therefore be hypothesized that coffee intake and exercise increases liver TG catabolism in trained rats, and at the same time, delays the recovery of hepatic TG levels.
Exercise alone and the exercise-training interaction significantly affected muscle TG concentrations. Effects of the exercise-training interaction on muscle TG was significant for controlled diet with coffee solution group (Fig. 4; P = 0.0256). The NTCF-DE group had significantly higher muscle TG levels compared to other experimental groups while TCF-BE animals showed significantly lower muscle TG levels. TG in the muscle is metabolized into FFAs that are used to generate ATP via β-oxidation in mitochondria. Our study demonstrated that training decreases muscle TG concentrations. Fatty acid utilization is particularly important during exercise when skeletal muscles must switch from using glucose to FFAs as their primary fuel source [37]. The present study indicates that training increases FFA levels and decreases liver TG concentrations at DE. Coffee intakes and exercises increase liver TG catabolism and FFA release in trained rats. Consistently, some antioxidants have been shown to increase the lipolysis of body fat [38,39]. In a past study, we investigated the effects of coffee intake and exercise on the anti-oxidative activity and plasma cholesterol profile of physically trained rats while they were exercising. And we also found out that coffee intake can increase the anti-oxidative defense system and decreasing HDL-cholesterol [40].
Protein catabolism has been reported to increase significantly when muscle glycogen storage are depleted by only 33-35% [41,42]. Since no differences in plasma, liver, or muscle protein levels were observed between the four groups under exercises in our study, protein appears to be a relatively minor source of energy that is not affected by moderate physical training.
To improve exercise capacity, it is important to reduce fatigue. This may be accomplished by providing energy substrates, enhancing energy-generating metabolic pathways, increasing cardiovascular and respiratory functions, increasing the size or number of energy-generating cells, eliminating fatigue-related metabolic byproducts, and preventing catabolism in energy-generating cells thereby demonstrating how certain nutrients or dietary supplements are believed to reduce fatigue [43-45]. The present study indicates that coffee intakes may influence training-induced modifications of fuel utilization and antioxidant defense system. The interaction of coffee and training significantly affects hemoglobin, hematocrit, and liver glycogen levels at DE and AE, and liver TG concentrations at DE and AE. Physical training made the animals adapt by increasing glycogen storage, but coffee consumption delayed the recovery of liver glycogen levels in trained rats after exercise. Training also increase FFA concentrations and decreases liver TG levels at DE. Exercising and coffee intakes stimulate FFA release and increase liver TG catabolism in trained rats.
Second, training was the main factor which increased heart weight and decreased visceral fat in this study. Increased heart weight is symbolizes physiological adaptation in response to both elevated blood pressure and increased ventricular filling due to training [46]. Reduced visceral fat is often associated with enhanced lipolysis, usually regular exercise reduces adipose tissue mass along with citrate synthetase activation in skeletal muscle [47].
Third, the current study demonstrated that coffee intakes and trainings decrease hemoglobin and hematocrit levels. Coffee may decrease the absorption of iron [48]. A further drop in hemoglobin levels after exercising could be explained by hemolysis due to the physical force generated during the exercise [49]. Therefore, coffee may have decreased iron absorption and the impact of running could have resulted in hemolysis.
By all accounts, results of this study indicate that coffee intakes can increase lipolysis during exercise while, also promoting the development of anemia and delaying carbohydrate replenishment. Physical training may delay the onset of fatigue and improve exercising performances by facilitating the mobilization and oxidation fat while conserving limited carbohydrate stores. Therefore, coffee appears to be an effective ergogenic aid, especially in terms of lipolysis, when administered at the appropriate time and dose.
Footnotes
This study was supported by a 2011 Research Grant from Duksung Women's University.
References
- 1.Green HJ. How important is endogenous muscle glycogen to fatigue in prolonged exercise? Can J Physiol Pharmacol. 1991;69:290–297. doi: 10.1139/y91-045. [DOI] [PubMed] [Google Scholar]
- 2.Hawley JA, Brouns F, Jeukendrup A. Strategies to enhance fat utilisation during exercise. Sports Med. 1998;25:241–257. doi: 10.2165/00007256-199825040-00003. [DOI] [PubMed] [Google Scholar]
- 3.Campbell I. Starvation, exercise, injury and obesity. Anaesth Intensive Care Med. 2004;5:243–248. [Google Scholar]
- 4.Choi EY, Cho YO. Moderate physical training can increase muscle glycogen levels but does not alter protein levels with exercise in rats. Nutr Sci. 2006;9:112–116. [Google Scholar]
- 5.Barnett C, Carey M, Proietto J, Cerin E, Febbraio MA, Jenkins D. Muscle metabolism during sprint exercise in man: influence of sprint training. J Sci Med Sport. 2004;7:314–322. doi: 10.1016/s1440-2440(04)80026-4. [DOI] [PubMed] [Google Scholar]
- 6.Stuewe SR, Gwirtz PA, Agarwal N, Mallet RT. Exercise training enhances glycolytic and oxidative enzymes in canine ventricular myocardium. J Mol Cell Cardiol. 2000;32:903–913. doi: 10.1006/jmcc.2000.1131. [DOI] [PubMed] [Google Scholar]
- 7.Murase T, Haramizu S, Shimotoyodome A, Tokimitsu I, Hase T. Green tea extract improves running endurance in mice by stimulating lipid utilization during exercise. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1550–R1556. doi: 10.1152/ajpregu.00752.2005. [DOI] [PubMed] [Google Scholar]
- 8.Saldanha Aoki M, Rodriguez Amaral Almeida AL, Navarro F, Bicudo Pereira Costa-Rosa LF, Pereira Bacurau RF. Carnitine supplementation fails to maximize fat mass loss induced by endurance training in rats. Ann Nutr Metab. 2004;48:90–94. doi: 10.1159/000077043. [DOI] [PubMed] [Google Scholar]
- 9.Rogers NL, Dinges DF. Caffeine: implications for alertness in athletes. Clin Sports Med. 2005;24:e1–e13. x–xi. doi: 10.1016/j.csm.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Spiller MA. The chemical components of coffee. In: Spiller GA, editor. Caffeine. Boca Raton, FL: CRC Press; 1998. [Google Scholar]
- 11.Costill DL, Dalsky GP, Fink WJ. Effects of caffeine ingestion on metabolism and exercise performance. Med Sci Sports. 1978;10:155–158. [PubMed] [Google Scholar]
- 12.Pasman WJ, van Baak MA, Jeukendrup AE, de Haan A. The effect of different dosages of caffeine on endurance performance time. Int J Sports Med. 1995;16:225–230. doi: 10.1055/s-2007-972996. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez de Sotillo DV, Hadley M, Sotillo JE. Insulin receptor exon 11+/- is expressed in Zucker (fa/fa) rats, and chlorogenic acid modifies their plasma insulin and liver protein and DNA. J Nutr Biochem. 2006;17:63–71. doi: 10.1016/j.jnutbio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Venables MC, Hulston CJ, Cox HR, Jeukendrup AE. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am J Clin Nutr. 2008;87:778–784. doi: 10.1093/ajcn/87.3.778. [DOI] [PubMed] [Google Scholar]
- 15.Arion WJ, Canfield WK, Ramos FC, Schindler PW, Burger HJ, Hemmerle H, Schubert G, Below P, Herling AW. Chlorogenic acid and hydroxynitrobenzaldehyde: new inhibitors of hepatic glucose 6-phosphatase. Arch Biochem Biophys. 1997;339:315–322. doi: 10.1006/abbi.1996.9874. [DOI] [PubMed] [Google Scholar]
- 16.Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr. 2003;78:728–733. doi: 10.1093/ajcn/78.4.728. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu M, Kobayashi Y, Suzuki M, Satsu H, Miyamoto Y. Regulation of intestinal glucose transport by tea catechins. Biofactors. 2000;13:61–65. doi: 10.1002/biof.5520130111. [DOI] [PubMed] [Google Scholar]
- 18.Tunnicliffe JM, Erdman KA, Reimer RA, Lun V, Shearer J. Consumption of dietary caffeine and coffee in physically active populations: physiological interactions. Appl Physiol Nutr Metab. 2008;33:1301–1310. doi: 10.1139/H08-124. [DOI] [PubMed] [Google Scholar]
- 19.Desbrow B, Hughes R, Leveritt M, Scheelings P. An examination of consumer exposure to caffeine from retail coffee outlets. Food Chem Toxicol. 2007;45:1588–1592. doi: 10.1016/j.fct.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, Logroscino G, Hu FB, van Dam RM. Coffee consumption and risk of stroke in women. Circulation. 2009;119:1116–1123. doi: 10.1161/CIRCULATIONAHA.108.826164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassid WZ, Abraham S. Chemical procedures for analysis of polysaccharides. Methods Enzymol. 1957;3:34–50. [Google Scholar]
- 22.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 23.Giegel JL, Ham AB, Clema W. Manual and semi-automated procedures for measurements of triglycerides in serum. Clin Chem. 1975;21:1575–1581. [PubMed] [Google Scholar]
- 24.Raabo E, Terkildsen TC. On the enzymatic determination of blood glucose. Scand J Clin Lab Invest. 1960;12:402–407. doi: 10.3109/00365516009065404. [DOI] [PubMed] [Google Scholar]
- 25.Rogiers V. Stability of the long chain non-esterified fatty acid pattern in plasma and blood during different storage conditions. Clin Chim Acta. 1978;84:49–54. doi: 10.1016/0009-8981(78)90475-8. [DOI] [PubMed] [Google Scholar]
- 26.Hargreaves M. Muscle glycogen and metabolic regulation. Proc Nutr Soc. 2004;63:217–220. doi: 10.1079/PNS2004344. [DOI] [PubMed] [Google Scholar]
- 27.Akerstrom TC, Birk JB, Klein DK, Erikstrup C, Plomgaard P, Pedersen BK, Wojtaszewski J. Oral glucose ingestion attenuates exercise-induced activation of 5'-AMP-activated protein kinase in human skeletal muscle. Biochem Biophys Res Commun. 2006;342:949–955. doi: 10.1016/j.bbrc.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 28.Greer F, Hudson R, Ross R, Graham T. Caffeine ingestion decreases glucose disposal during a hyperinsulinemic-euglycemic clamp in sedentary humans. Diabetes. 2001;50:2349–2354. doi: 10.2337/diabetes.50.10.2349. [DOI] [PubMed] [Google Scholar]
- 29.Keijzers GB, De Galan BE, Tack CJ, Smits P. Caffeine can decrease insulin sensitivity in humans. Diabetes Care. 2002;25:364–369. doi: 10.2337/diacare.25.2.364. [DOI] [PubMed] [Google Scholar]
- 30.Clifford MN. Chlorogenic acids and other cinnamates - nature, occurrence and dietary burden. J Sci Food Agric. 1999;79:362–372. [Google Scholar]
- 31.Herling AW, Burger H, Schubert G, Hemmerle H, Schaefer H, Kramer W. Alterations of carbohydrate and lipid intermediary metabolism during inhibition of glucose-6-phosphatase in rats. Eur J Pharmacol. 1999;386:75–82. doi: 10.1016/s0014-2999(99)00748-7. [DOI] [PubMed] [Google Scholar]
- 32.McCarty MF. A chlorogenic acid-induced increase in GLP-1 production may mediate the impact of heavy coffee consumption on diabetes risk. Med Hypotheses. 2005;64:848–853. doi: 10.1016/j.mehy.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 33.Ma YT. Biomedical Acupuncture for Sports and Trauma Rehabilitation:Dry Needling Techniques. St. Louis, MO: Churchill Livingstone/Elsevier; 2011. Homeostasis and stress in sports and exercise; pp. 6–19. [Google Scholar]
- 34.Casal DC, Leon AS. Failure of caffeine to affect substrate utilization during prolonged running. Med Sci Sports Exerc. 1985;17:174–179. doi: 10.1249/00005768-198502000-00029. [DOI] [PubMed] [Google Scholar]
- 35.Astrup A, Breum L, Toubro S, Hein P, Quaade F. The effect and safety of an ephedrine/caffeine compound compared to ephedrine, caffeine and placebo in obese subjects on an energy restricted diet. A double blind trial. Int J Obes Relat Metab Disord. 1992;16:269–277. [PubMed] [Google Scholar]
- 36.van den Berghe G. The role of the liver in metabolic homeostasis: implications for inborn errors of metabolism. J Inherit Metab Dis. 1991;14:407–420. doi: 10.1007/BF01797914. [DOI] [PubMed] [Google Scholar]
- 37.Wende AR, Huss JM, Schaeffer PJ, Giguère V, Kelly DP. PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol. 2005;25:10684–10694. doi: 10.1128/MCB.25.24.10684-10694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-β-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003;133:2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- 39.Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: from bedside to bench. Mol Nutr Food Res. 2006;50:176–187. doi: 10.1002/mnfr.200500102. [DOI] [PubMed] [Google Scholar]
- 40.Choi EY, Jang JY, Cho YO. Coffee intake can promote activity of antioxidant enzymes with increasing MDA level and decreasing HDL-cholesterol in physically trained rats. Nutr Res Pract. 2010;4:283–289. doi: 10.4162/nrp.2010.4.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemon PW. Is increased dietary protein necessary or beneficial for individuals with a physically active lifestyle? Nutr Rev. 1996;54:S169–S175. doi: 10.1111/j.1753-4887.1996.tb03913.x. [DOI] [PubMed] [Google Scholar]
- 42.Andersen LL, Tufekovic G, Zebis MK, Crameri RM, Verlaan G, Kjaer M, Suetta C, Magnusson P, Aagaard P. The effect of resistance training combined with timed ingestion of protein on muscle fiber size and muscle strength. Metabolism. 2005;54:151–156. doi: 10.1016/j.metabol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Lemmen HJ, Alderliesten RC, Benedictus R. Fatigue initiation behaviour throughout friction stir welded joints in AA2024-T3. Int J Fatigue. 2010;32:1928–1936. [Google Scholar]
- 44.Ma YT. Biomedical Acupuncture for Sports and Trauma Rehabilitation: Dry Needling Techniques. St. Louis, MO: Churchill Livingstone/Elsevier; 2011. Human brain plasticity, sports, and sports injuries; pp. 20–25. [Google Scholar]
- 45.You L, Zhao M, Regenstein JM, Ren J. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem. 2011;124:188–194. doi: 10.1021/jf2016368. [DOI] [PubMed] [Google Scholar]
- 46.Sedehi D, Ashley EA. Defining the limits of athlete's heart: implications for screening in diverse populations. Circulation. 2010;121:1066–1068. doi: 10.1161/CIR.0b013e3181d7308a. [DOI] [PubMed] [Google Scholar]
- 47.Irving BA, Davis CK, Brock DW, Weltman JY, Swift D, Barrett EJ, Gaesser GA, Weltman A. Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc. 2008;40:1863–1872. doi: 10.1249/MSS.0b013e3181801d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morck TA, Lynch SR, Cook JD. Inhibition of food iron absorption by coffee. Am J Clin Nutr. 1983;37:416–420. doi: 10.1093/ajcn/37.3.416. [DOI] [PubMed] [Google Scholar]
- 49.Peeling P, Dawson B, Goodman C, Landers G, Wiegerinck ET, Swinkels DW, Trinder D. Cumulative effects of consecutive running sessions on hemolysis, inflammation and hepcidin activity. Eur J Appl Physiol. 2009;106:51–59. doi: 10.1007/s00421-009-0988-7. [DOI] [PubMed] [Google Scholar]


