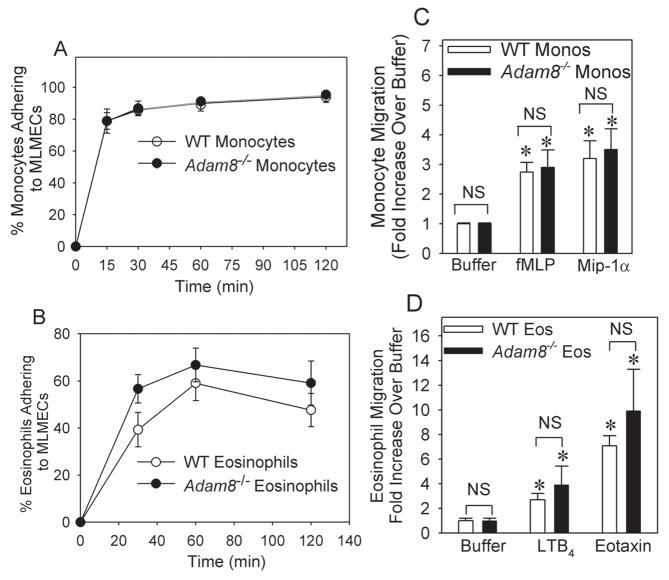
Fig. 6. Adam8 is not required for murine eosinophils or monocytes to adhere to lung microvascular endothelial cell (LMVEC) monolayers or migrate in vitro.
In A, equal numbers (106 cells) of bonemarrow-derived monocytes isolated from unchallenged BALB/c WT or BALB/c Adam8−/− mice were incubated at 37°C for 1 h in duplicate with or without 1 μg/ml LPS for 2 h on LMVEC monolayers that had been pre-incubated for 4 h at 37°C with or without 10 ng/ml Tnf-α. In B, equal numbers (106 cells) of bone marrow-derived eosinophils isolated from unchallenged BALB/c WT and BALB/c Adam8−/− mice were incubated in duplicate at 37°C with or without 10−7M LTC4 on LMVEC monolayers that had been pre-incubated for 18 h with or without 10−7M interleukin-4 and 10−7M interleukin-13. In A and B, the percentage of leukocytes adhering to the LMVEC monolayers was determined. Data are mean ± SEM; n= 3–4 experiments. In C and D, we used Boyden micro-chemotaxis assay chambers to compare the migration of bone-marrow derived WT vs. Adam8−/− monocytes to buffer alone, 10−7M fMLP, or 10−7 M Mip-1α in the lower chambers (in C) or the migration of WT vs. Adam8−/− bone marrow derived eosinophils in response to buffer alone, 10−7 M LTB4, or 10−8M murine eotaxin in the lower chambers (in D). Data are expressed as fold increase in cellular migration when compared with cellular migration in response to buffer alone. Data are mean ± SEM from 3–6 wells per experimental condition and 3–6 separate experiments. Asterisk indicates p ≤ 0.031 versus cells in C and p ≤ 0.01 in D when compared with rates of migration of cells of the same genotype in response to buffer.