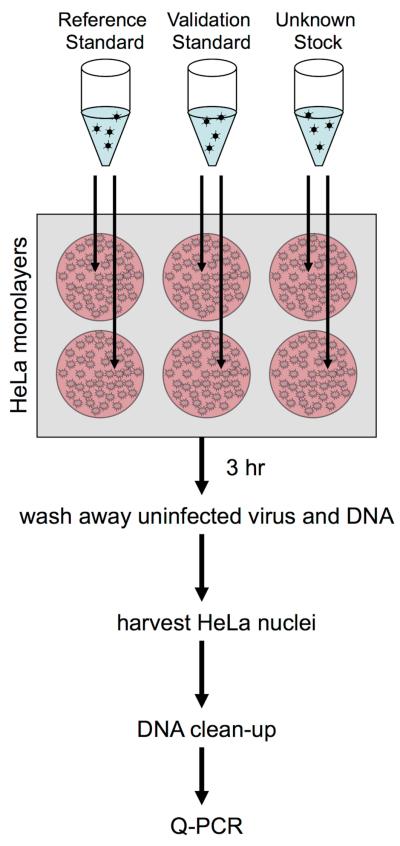
Figure 2. Overview of the Infectious Genome Protocol.
Each stock of adenovirus to be titered was used to infect replicate wells of HeLa cell monolayers in 6-well tissue culture plates. 5 μL of vector stock (crude or purified) was diluted into 500 μL of serum-free media and overlaid on the monolayer for three to six hours at 37°C. The inoculant was aspirated, and monolayers were washed twice with PBS. Cell membranes, but not nuclear membranes, were lysed in place by the addition of NP-40 detergent. The nuclei were pelleted, and used to prepare total DNA by means of a commercial kit. The resulting DNA was then used as template in Q-PCR to quantify the number of vector genomes that had translocated to the nucleus during the incubation period. The titer of each stock was then determined relative to a reference standard stock of pre-established titer that was processed in parallel. Additionally, two validation stocks of pre-established titer were processed in parallel in order to identify errors during processing.