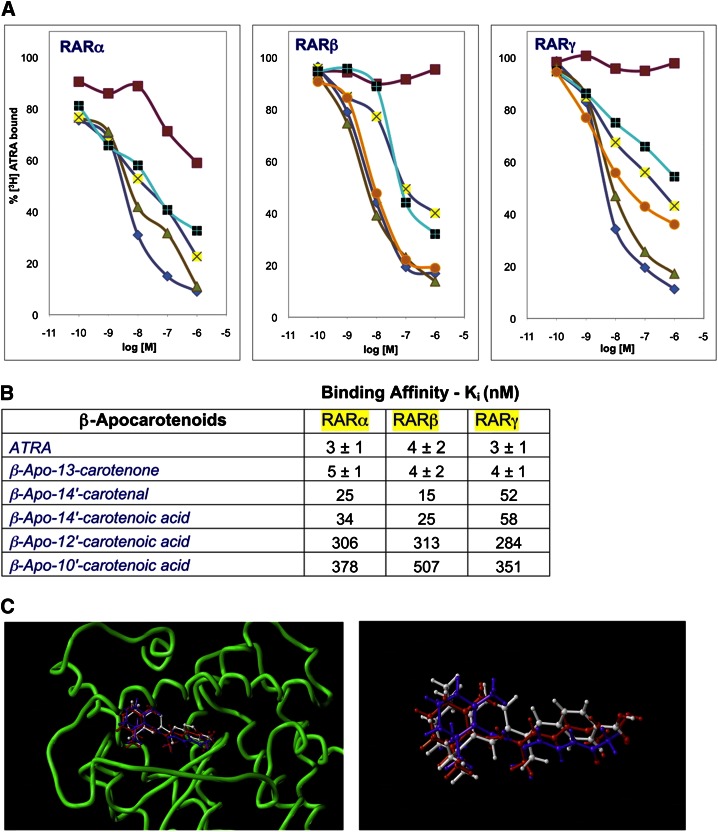
Fig. 4.
β-Apo-13-carotenone is a high-affinity ligand for purified retinoic acid receptors and fits into the ligand binding site. (A) Competitive displacement of 5 nM tritiated atRA from purified RAR proteins by unlabeled atRA (filled diamond) as a positive control, β-apo-13-carotenone (filled triangle), 14′-CA (plus), 14′-AL (cross), and 13-cis-RA (filled square) as a negative control for RARα (left) experiment, and CD 2665 (filled circle) and retinyl acetate (filled square) as a negative control for RARβ (middle) and RARγ (right) experiments. Points shown are means of n = 3 with a variance of less than 10%. (B) Binding affinities (in nM) of β-apocarotenoids to RARs calculated from the data shown in (A) and additional experiments with β-apo-12′-and β-apo-10′-carotenoic acids. For atRA and β-apo-13-carotenone, variance shown is for three independent experiments. (C) Molecular modeling of the docking of atRA (red) and β-apo-13-carotenone (purple) into the ligand-binding site (protein backbone in green) of RARβ (PDB entry:1xap) (left). Shown on the right is the energy minimized then docked conformations of atRA (red) and β-apo-13-carotenone (purple) overlaid onto the conformation of the agonist TTNPB (white) as observed in the X-ray structure (177).