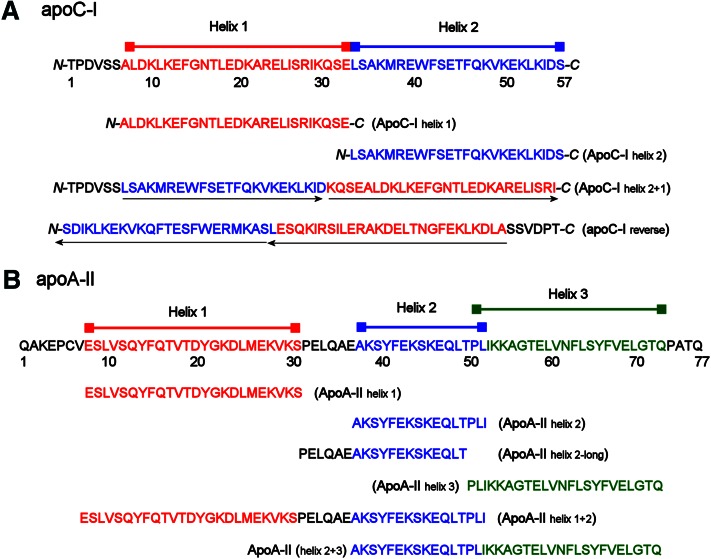
Fig. 3.
Synthetic peptides reflecting individual helical domains of apoC-I and apoA-II. (A) Full-length sequence of human mature apoC-I. Consensus helical domains based on sequence analyses are indicated, as are two bihelical constructs. The first simply reverses the order of the helices in the WT sequence (maintaining a predicted turn sequence KQSE between the helices), while the second completely reverses the amino acid order of the WT protein. The orientations of the helices in these two mutants with respect to the WT protein are shown by the black arrows under the sequences. (B) Full-length sequence of human mature (monomeric) apoA-II. Consensus helical domains based on several sequence analyses (see text) are shown in red, blue, and green, with correspondingly colored bars above. Individual peptides designed to mimic each individual helix (including two possibilities for helix 2) are aligned under the sequence. The sequence of two bihelical constructs is also shown.