Abstract
INTRODUCTION
Fistulas are a relatively common occurrence in Crohn's disease (CD), and often present early in the disease process. Additionally, patients suffering from either CD or ulcerative colitis are shown to have an increased risk of colorectal malignancies compared with the general population.
PRESENTATION OF CASE
We present a case of adenocarcinoma in an ano-vaginal fistula in a patient with longstanding CD.
DISCUSSION
Various pathogenic mechanisms for the development of carcinoma in fistulas have been suggested, but there is no consensus and indeed this risk may be cumulative. In this case report, we also discuss the pathogenesis of mucinous adenocarcinoma in fistulas secondary to CD.
CONCLUSION
Better detection of adenocarcinoma in patients presenting with persistent non-resolving fistulas in the presence of CD should be undertaken with regular biopsies following examinations under anaesthetic of the anorectum.
Keywords: Crohn's disease, Fistulae, Mucinous, Adenocarcinoma
1. Introduction
Crohn's disease (CD) is known to be associated with perianal disease in the form of fistulas and abscesses. The risk of malignancy with CD is also well recognised however the location is variable and not always predictable. A case of a mucinous adenocarcinoma at an unusual location is presented here.
2. Presentation of case
A 53-year-old female with a 30-year history of Crohn's disease (CD), and previous ileo-colic resection 15 years before, developed new right-sided abdominal symptoms. She was referred to a gastroenterologist by the general practitioner. Physical examination to exclude perianal sepsis before starting cyotokine-modulating monoclonal antibody therapy showed a 2 cm × 4 cm perianal lump at the anal verge in the 6 o’clock position. Her disease had been quiescent in the period since her resection but in the months before presenting to us with the perianal lesion, she was treated with azathioprine and a reducing dose of prednisolone. Her past medical history included cervical cancer 16 years prior, which was treated with surgery and radiotherapy (the notes for which were not able to be retrieved from the institution where she was treated due to its closure). She had no obstetric history or history of human immunodeficiency syndrome and smoked 5 cigarettes per day.
A magnetic resonance image (MRI) of the abdomen and pelvis following identification of the lesion demonstrated a complex fistula with an inter-sphincteric fluid collection on the left side in the mid anal canal. This communicated with a ‘Y’ shaped defect extending into the perineal body and vaginal introitus. In addition, a separate 2.7 cm cystic collection representing the lump seen clinically was identified superficially in the midline of the natal cleft, posterior to the anal canal (Fig. 1). In the abdomen, there was clear evidence of active colitis affecting the majority of the colon and short segment of the neo-terminal ileum. Colonoscopy revealed a neo-terminal ileal stricture, which histologically showed ulceration and evidence of active chronic colitis with rectal sparing, but no evidence of malignancy. A computed tomography (CT) scan of the chest, abdomen and pelvis did not show any evidence of metastatic disease.
Fig. 1.

The T2 images MRI of the abdomen and pelvis showing (a) the cystic collection in the midline of the natal cleft and (b) the ‘Y’ shaped fluid connection tract.
She subsequently underwent an examination under anaesthetic (EUA) of the anorectum and excision biopsy of the perianal lesion, which connected with the fistula at the 5 o’clock position (Fig. 2). At this time the fistula was not found to connect with the lower posterior vagina. The histopathology of the excised lump showed a mucin-filled cyst lined with dysplastic epithelium and a second opinion was sought to review the histology. In the opinion of one pathologist (NS), this represented a mucinous well-differentiated adenocarcinoma arising in a fistula in the context of chronic inflammatory bowel disease (Fig. 3).
Fig. 2.

The perianal lesion (a) before and (b) after excision.
Fig. 3.
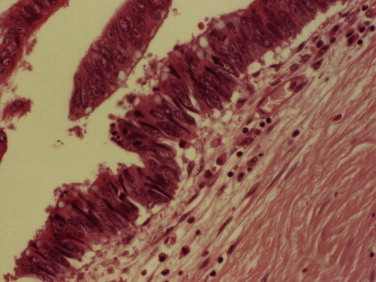
A photomicrograph of the histological specimen of the perianal lesion showing dysplastic colorectal type epithelium in the fistula tract under high power magnification.
Management options were discussed with the patient including the need for surgery for her reactivated ileo-colonic CD. The option of radiotherapy was discussed, however the multi-disciplinary team (MDT) and the patient were keen to avoid this given the unknown previous treatment for the cervical cancer and the risks of radiation enteritis in a Crohn's patient which may then predispose to short gut syndrome. In more than one MDT meeting, together with the patient, the decision was made to perform an open ileo-panproctocolectomy with a wide ischioanal fossa excision and posterior vaginectomy with a perianal reconstruction using a pedicled rectus abdominis flap.
An EUA of the rectum prior to laparotomy revealed the secretion of mucin on digital rectal examination (Fig. 4). The patient underwent an ileo-panproctocolectomy with a left vertical rectus abdominis (VRAM) reconstruction (as there was a right sided scar from previous surgery), small bowel resection, right salphingectomy, posterior vaginectomy and ileostomy. The small bowel resection was necessitated by the presence of a Crohn's mass involving the right fallopian tube. Post-operatively she required high dependency unit care, and had a number of complications including non-healing of the end ileostomy and enteric content contaminated the extensive dissection cavity associated with the left VRAM donor site. This was drained surgically and healed over a number of weeks. She was readmitted within a month with acute renal failure due to a high output ileostomy and then with a pulmonary embolus 6 weeks post-operatively despite being on prophylactic treatment with low molecular weight heparin during her hospital stay.
Fig. 4.

The examination under anaesthetic of the anorectum at the time of resection showing evidence of residual fistula with mucin expressible from the vaginal end of the fistula.
The final histology of the resected specimen revealed an ano-vaginal fistula tract centrally lined with colorectal type dysplastic epithelium, which formed well-differentiated glands producing mucin. This was positive for cytokeratin (CK) 20, MNF116 antibody and negative for CK7. There was also evidence of focal high-grade dysplasia within the anal sphincter. The rectal mucosa showed chronic inflammatory changes and there was no evidence of anal squamous dysplasia. The final surgical pathology diagnosis after assessment by two consultant histopathologists (AA and NS) was a well-differentiated mucinous adenocarcinoma (estimated size of at least 35 mm). The small bowel mass showed serositis and some granulomas in keeping with CD. The margins were clear with no lymph node involvement. Hence, the patient did not require any further adjuvant therapy.
3. Discussion
Both ano-vaginal and recto-vaginal fistulas are known to occur in patients with CD. They occur in approximately a quarter of females with CD.1 The processes behind these have been suggested to be due to factors such as the inflammatory nature of the disease and transmural rectal involvement.2,3 Moreover, malignancy complicates long standing CD, thought to be secondary to constant mucosal regeneration which could lead to dysplasia and subsequently neoplasia.4,5 An incidence of 0.7 for carcinoma arising in a fistula in CD has been reported from the patient series of Ky and colleagues.4 CD as with ulcerative colitis (UC) is known to be associated with the risk of developing colorectal cancer (CRC). The risk in CD can be up to 6-fold that of the general population for developing CRC and is recognised to be associated with a long duration of disease.6
Ano-rectal fistulas in CD have been reported as occurring between 25 and 60 percent in disease affecting the terminal ileum and rectum respectively.4 However malignant transformation complicating an anal fistula is very rare. Not unexpectedly the average age of presentation for a fistula with CD is relatively early compared to the presentation of carcinoma.4 The reported cases of carcinoma arising from CD fistulas have been in patients with CD for a period of approximately 20 years,4,7 as in the case of our patient. Early reports have been made about recto-vaginal fistulas occurring more commonly in severe colonic CD.8 A case matched study of perianal fistulous CD with and without CRC by Sobala et al.9 showed a significant association of cancer in long standing fistulas for a median period of 11 years. This was similar to the findings of a systematic review of malignant cases of CD fistulas.10
The pathogenesis of fistulas in general is unknown. The development of carcinoma arising in CD fistulas was initially thought to be squamous cell beginning from the squamous epithelium of the fistula tract. Suggestions have also been made of origins of the mucinous adenocarcinomas originating from the anal glands.4 However, in the case presented here there was no histological evidence of squamous cell invasion or direct involvement of the anal glands leading to the development of carcinoma. The origins of adenocarcinomas associated with fistulas in CD have been debatable. Nishigami et al.11 performed immuno-histological studies using CK20 and mucins and established adenocarcinomas in CD fistulas are associated with rectal type mucosa. Our immuno-histochemical findings also support this.
Our case is similar to that of Smith et al.7 who reported a perianal mass in an elderly woman with extensive perianal and vulvar CD, that revealed a mucinous adenocarcinoma. The authors suggested the colonic epithelialization of the fistula tract might have dysplastic potential that subsequently leads to development of mucinous adenocarcinoma without an underlying rectal cancer. Mucinous carcinomas in CD in a fistula tract have been reported.4,5,7,12–14 In the case presented by Freeman et al.,15 the patient had recurrent episodes of perianal and rectal fistulas, which was not the situation in our patient. A previously reported case of mucinous carcinoma occurring in a fistula tract in CD was thought to be associated with immunosuppressants such as azathioprine.10
In our case, cancer had originated de novo in a CD fistula without rectal or anal mucosa involvement. Perhaps, our patient may have an additional predisposition to cancer because of her previous diagnosis of cervical cancer and possibly human papilloma virus exposure. The effects of immunosuppressive drugs cannot also be discounted as possibly playing a role. Persistent non-healing fistulas in CD should alert the clinician to underlying malignancy.16
4. Conclusion
Thorough surveillance of the perianal region and fistula tracts should be incorporated with an EUA and biopsy in patients with perianal CD especially when there is no clinical suspicion of malignancy.
Conflict of interest statement
There are no conflicts of interest to disclose.
Funding
No funding has been received for this manuscript.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
MAW, AAg drafted the manuscript; AA, YM and AM provided significant advice in writing the manuscript. AM conceived the idea of writing the case report. All authors read and approved the final manuscript.
Acknowledgement
Dr. Nigel Scott, Consultant Pathologist, at St James University Hospital, Leeds, UK for the review of the histology slides.
References
- 1.Heyen F., Winslet M.C., Andrews H., Alexander-Williams J., Keighley M.R. Vaginal fistulas in Crohn's disease. Diseases of the Colon and Rectum. 1989;32(5):379–383. doi: 10.1007/BF02563688. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberger D.A., Goldberg S.M. The management of rectovaginal fistulas. Surgical Clinics of North America. 1983;63(1):61–79. doi: 10.1016/s0039-6109(16)42930-0. [DOI] [PubMed] [Google Scholar]
- 3.Radcliffe A.G., Ritchie J.K., Hawley P.R., Lennard-Jones J.E., Northover J.M. Anovaginal and rectovaginal fistulas in Crohn's disease. Diseases of the Colon and Rectum. 1988;31(2):94–99. doi: 10.1007/BF02562636. [DOI] [PubMed] [Google Scholar]
- 4.Ky A., Sohn N., Weinstein M.A., Korelitz B.I. Carcinoma arising in anorectal fistulas of Crohn's disease. Diseases of the Colon and Rectum. 1998;41(8):992–996. doi: 10.1007/BF02237388. [DOI] [PubMed] [Google Scholar]
- 5.Laurent S., Barbeaux A., Detroz B., Detry O., Louis E., Belaiche J. Development of adenocarcinoma in chronic fistula in Crohn's disease. Acta Gastroenterologica Belgica. 2005;68(1):98–100. [PubMed] [Google Scholar]
- 6.Weedon D.D., Shorter R.G., Ilstrup D.M., Huizenga K.A., Taylor W.F. Crohn's disease and cancer. The New England Journal of Medicine. 1973;289(21):1099–1103. doi: 10.1056/NEJM197311222892101. [DOI] [PubMed] [Google Scholar]
- 7.Smith R., Hicks D., Tomljanovich P.I., Lele S.B., Rajput A., Dunn K.B. Adenocarcinoma arising from chronic perianal Crohn's disease: case report and review of the literature. The American Surgeon Journal. 2008;74(1):59–61. [PubMed] [Google Scholar]
- 8.Bagby R.J., Clements J.L., Jr., Patrick J.W., Rogers J.V., Weens H.S. Genitourinary complications of granulomatous bowel disease. American Journal of Roentgenology, Radium Therapy and Nuclear Medicine. 1973;117(2):297–306. doi: 10.2214/ajr.117.2.297. [DOI] [PubMed] [Google Scholar]
- 9.Sobala A., Herbst F., Novacek G., Vogelsang H. Colorectal carcinoma and preceding fistula in Crohn's disease. Journal of Crohn's and Colitis. 2010;4(2):189–193. doi: 10.1016/j.crohns.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Thomas M., Bienkowski R., Vandermeer T.J., Trostle D., Cagir B. Malignant transformation in perianal fistulas of Crohn's disease: a systematic review of literature. Journal of Gastrointestinal Surgery: Official Journal of the Society for Surgery of the Alimentary Tract. 2010;14(1):66–73. doi: 10.1007/s11605-009-1061-x. [DOI] [PubMed] [Google Scholar]
- 11.Nishigami T., Kataoka T.R., Ikeuchi H., Torii I., Sato A., Tomita N. Adenocarcinomas associated with perianal fistulas in Crohn's disease have a rectal, not an anal, immunophenotype. Pathology. 2011;43(1):36–39. doi: 10.1097/PAT.0b013e328340e4d6. [DOI] [PubMed] [Google Scholar]
- 12.Chaikhouni A., Regueyra F.I., Stevens J.R. Adenocarcinoma in perineal fistulas of Crohn's disease. Diseases of the Colon and Rectum. 1981;24(8):639–643. doi: 10.1007/BF02605765. [DOI] [PubMed] [Google Scholar]
- 13.Melichar B., Bures J., Dedic K. Anorectal carcinoma after infliximab therapy in Crohn's disease: report of a case. Diseases of the Colon and Rectum. 2006;49(8):1228–1233. doi: 10.1007/s10350-006-0647-6. [DOI] [PubMed] [Google Scholar]
- 14.Zagoni T., Peter Z., Sipos F., Dichazi C., Tarjan Z., Dobo I. Carcinoma arising in enterocutan fistulas of Crohn's disease patients: description of two cases. International Journal of Colorectal Disease. 2006;21(5):461–464. doi: 10.1007/s00384-005-0028-y. [DOI] [PubMed] [Google Scholar]
- 15.Freeman H.J., Perry T., Webber D.L., Chang S.D., Loh M.Y. Mucinous carcinoma in Crohn's disease originating in a fistulous tract. World Journal of Gastrointestinal Oncology. 2010;2(7):307–310. doi: 10.4251/wjgo.v2.i7.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inamdar N.V., Schwarz P., Bailey H.R., Skibber J.M., Rich T.A., Sellin J. Development of mutinous adenocarcinoma in chronic Crohn's disease fistulas without luminal involvement. Inflammatory Bowel Diseases. 1995;1(4):280–283. doi: 10.1097/00054725-199512000-00006. [DOI] [PubMed] [Google Scholar]


