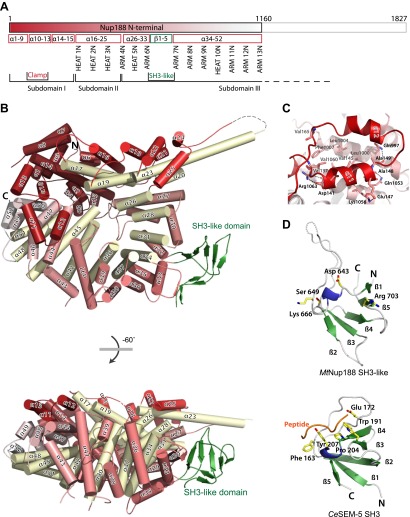
Figure 1. Crystal structures of Nup188N.
(A) Schematic diagram of full-length Myceliophthora thermophila Nup188N with details about the subdomain arrangement. The crystallized fragment is boxed and gradient-colored in red with helical HEAT- and ARM-repeats indicated. (B) The crystal structure of Nup188N is shown in cartoon representation. The helices are gradient-colored as in (A). The inner helical ring is in pale-yellow to highlight the superhelical organization of the protein. The SH3-like domain insert is shown in green. (C) Close-up of the ring-closing latch. The clamping helices α11 and α12 (red) contact a substantial surface area formed by helices 44, 46, and 47. (D) The SH3-like domain of Nup188 compared to the canonical, peptide-bound SH3 domain of Sem-5 from C. elegans (PDB 1SEM). Conserved residues important for peptide interaction are labeled. Note that these residues are not conserved in the SH3-like domain of Nup188. In addition, the SH3-like domain is a circular permutation of the canonical SH3-domain.