Abstract
Consumption of green tea (Camellia sinensis) may provide protection against chronic diseases, including cancer. Green tea polyphenols are believed to be responsible for this cancer preventive effect, and the antioxidant activity of the green tea polyphenols has been implicated as a potential mechanism. This hypothesis has been difficult to study in vivo due to metabolism of these compounds and poor understanding of the redox environment in vivo. Green tea polyphenols can be direct antioxidants by scavenging reactive oxygen species or chelating transition metals as has been demonstrated in vitro. Alternatively, they may act indirectly by up-regulating phase II antioxidant enzymes. Evidence of this latter effect has been observed in vivo, yet more work is required to determine under which conditions these mechanisms occur. Green tea polyphenols can also be potent pro-oxidants, both in vitro and in vivo, leading to the formation of hydrogen peroxide, the hydroxyl radical, and superoxide anion. The potential role of these pro-oxidant effects in the cancer preventive activity of green tea is not well understood. The evidence for not only the antioxidant, but also pro-oxidant, properties of green tea are discussed in the present review.
Keywords: Camellia sinensis, (–)-Epigallocatechin-3-gallate, Green Tea, Antioxidant, Cancer
1 Introduction
Green tea (Camellia sinensis, Theaceae) is the most widely consumed beverage, following water [1], and may have cancer preventive effects in vivo. Polyphenols in green tea are thought to be responsible for the cancer preventive effects observed in laboratory and epidemiological studies. Daily intake of polyphenols from green tea is high in some countries. Roughly 34 % of the total polyphenol consumption from beverages in Japan comes from green tea [2].
The green tea phenolic compounds of highest concentration are gallic acid (GA), (–)-gallocatechin (GC), (+)-catechin (C), (–)-epicatechin (EC), (–)-epigallocatechin (EGC), (–)-epicatechin gallate (ECG), (–)-epigallocatechin gallate (EGCG), p-coumaroylquinic acid (CA), and (–)-gallocatechin-3-gallate (GCG) (Fig 1), with EGCG being the most abundant by weight [3, 4]. Green tea also contains condensed and hydrolyzable tannins [5]. Green tea has the highest concentration of polyphenols compared to other teas, including EGCG, which may be why green tea can induce apoptotic cell death in cancer better than other teas [6].
Figure 1.
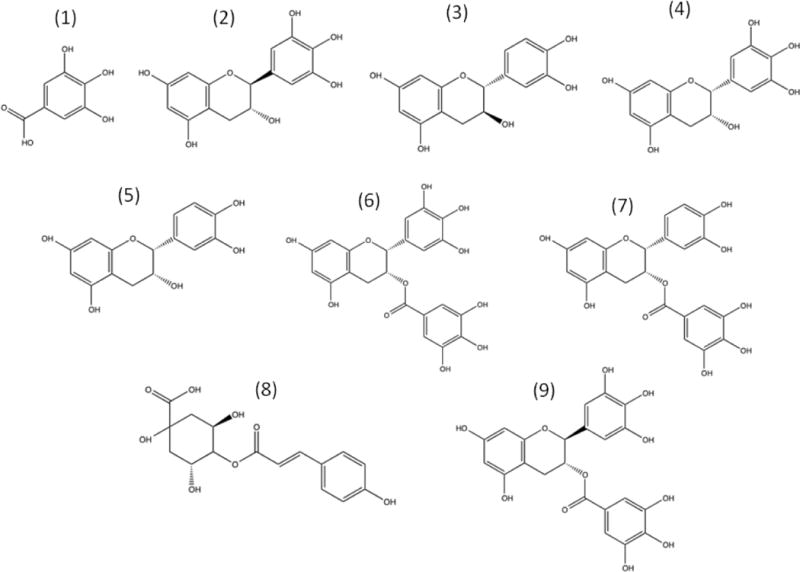
Chemical structures of the major green tea polyphenols. Structures shown: (1) Gallic acid, (2) (–)-gallocatechin, (3) (+)-catechin, (4) (–)-epigallocatechin, (5) (–)-epicatechin, (6) (–)-epigallocatechin gallate, (7) (–)-epicatechin gallate, (8) p-coumaroylquinic acid, and (9) (–)-gallocatechin gallate.
The extraction of green tea polyphenols into tea is both time and temperature dependent [3, 4]. Tea preparation is important, as hot water preparation causes tea to be better at scavenging oxidative radicals than cold water preparations [7], which is likely due to greater extraction of polyphenols. Green tea polyphenols can act as pro-oxidants by generating hydrogen peroxide. Adding milk to green tea decreases formation of hydrogen peroxide, independent of the presence of catalase [8], which decomposes hydrogen peroxide into water and oxygen. It could be that the polyphenols in green tea bind to proteins in milk, thereby inhibiting hydrogen peroxide production. Under oxidative conditions polymerization of green tea polyphenols can also occur [9].
The evidence for the potential anti-cancer effects of green tea effects in vivo is based, in part, on epidemiological studies. For instance, an inverse association exists between tea consumption and lung cancer for smokers but not nonsmokers [10], suggesting that green tea consumption may be more important for cancer prevention in high-risk populations. This is also evident in women that are at a higher risk of breast cancer due to a genetic predisposition, where green tea, but not black tea, consumption is associated with reduced risk of breast cancer [11]. Other inverse relationships that exist between green tea consumption and cancer risk include stomach cancer [12] and ovarian cancer [13]. Despite these numerous studies, the role of green tea consumption in the prevention of human cancer remains unclear, in part because there is a lack of data from controlled intervention studies.
Green tea and green tea polyphenols have been shown to have anti-cancer activity in a number of laboratory studies, which could be mediated through antioxidant or pro-oxidant mechanisms. Green tea polyphenols such as EGCG inhibit cell viability and induce apoptosis in a number of cancer cell lines such as osteogenic sarcoma [14], lymphoblastoid cells [15], leukemia cells [16], melanoma cells [17], T lymphocytes [18], and larynx carcinoma [19]. EGC can inhibit breast cancer cell viability through induction of apoptosis, yet not in normal breast cells [20]. Apoptosis by green tea polyphenols may occur independent of caspase-3 induction, through activation of p53 [19]. Evidence for cell cycle modulation also exists. EGCG in green tea causes a reduction in cell viability through G1 growth arrest in human breast cancer cells [21, 22], which likely occurs through suppression of cyclin D1 [17]. Green tea polyphenols can even cause differentiation of cancer cells into slower proliferating cells [23].
Inflammation is often a precursor to cancer. EGCG and ECG (but not EGC or EC) have anti-inflammatory effects as they induce apoptosis in monocytes at concentrations of 10 to 50 μM [24]. Interestingly, EGCG and ECG form the hydroxyl radical measured by electron spin resonance (ESR) but not by EC or EGC at neutral pH [25], indicating that the gallate group plays an important role in activity. EGCG also strongly inhibits various cancer cell lines more than other green tea polyphenols [26], and appears to have no effect in normal cells [17, 27]. This could be due to greater oxidative stress in cancer cells by EGCG than in normal cells, based on lower catalase levels in cancer cells [28]. Other effects of green tea polyphenols include inhibition of DNA synthesis in cancer cells and peroxyl radical generation [27]. Selenium could enhance anticancer activity of green tea [29], possibly by enhancing antioxidant activity [30, 31], or even its pro-oxidant activity [32].
Green tea polyphenols also have shown anti-cancer activity in vivo, yet the involvement of oxidative or antioxidative mechanisms is unclear. Green tea reduces tumor burden in a breast cancer rat model [21], and green tea polyphenols can reduce tumor burden in the forestomach of rats [25]. As in the in vitro studies, EGCG is the primary focus for the activity behind green tea consumption. EGCG can inhibit cancer in animal models [16, 33]. It also can reduce inflammation in the colon, causing a decrease in oxidative and inflammatory markers in a colitis rat model [34].
The basis for anticancer properties of green tea polyphenols in vitro and in vivo could due to their antioxidant or pro-oxidant properties (Fig 2). Due to conflicting evidence, it is likely that the specific oxidative mechanism depends on the type of cancer and environment surrounding cancer cells. The antioxidant and pro-oxidant mechanisms of green tea polyphenols will be discussed within this review, along with subsequent anti-cancer effects on markers of oxidative stress, cell proliferation, apoptosis, metastasis, inflammation, and tumor formation, in both in vitro and in vivo models of cancer.
Figure 2.
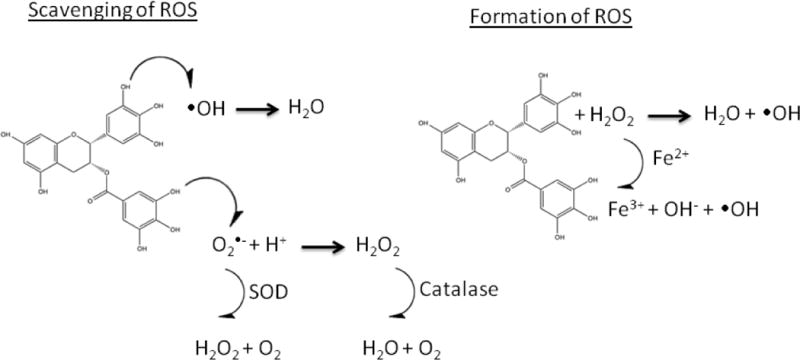
Scavenging and formation of ROS mechanisms by green tea polyphenols. These mechanisms are applicable to all green tea polyphenols, but (–)-Epigallocatechin gallate (6) is shown as a representative molecule.
2 Direct antioxidant effects of green tea
2.1 In vitro effects
Green tea polyphenols scavenge reactive oxygen species (ROS) by generating more stable phenolic radicals. The radical scavenging ability of EGCG has been a focus of many studies due to high relative concentrations in green tea and presence of the galloyl group on the B and D ring. Electron paramagnetic resonance (EPR) spectroscopy has revealed that EGCG reacts with O2– leading to oxidation of the D ring [35]. EPR has also shown that EGCG can scavenge OH and O2–[36].
A collection of assays have been developed and widely used to assess the radical scavenging/antioxidant activities of solutions, including green tea. One such assay is the ferric reducing/antioxidant power (FRAP) assay. A positive correlation exists between the phenolic content in green tea and the antioxidant activity measured by FRAP. In addition, green tea possesses more antioxidant capacity on average than Oolong and black teas (as measured by FRAP) [37]. The oxygen radical absorbance capacity (ORAC) assay is also commonly utilized to measure the antioxidant capacity of tea. Units are expressed as trolox equivalents, and green tea provides approximately 1300 μmol of trolox equivalents per gram of dried tea leaves [38]. Like in the FRAP assay, a positive correlation exists between ORAC values and green tea polyphenol content [39]. The Folin-Ciocalteu assay for total phenolic content has been used to show that green tea contains roughly 300 mg/g (expressed as EGCG) of dried leaves [38]. A positive correlation exists for other antioxidant capacity methods such as copper reducing power, DPPH scavenging, and superoxide scavenging with the total polyphenol content of tea measured by Folin-ciocalteu [2]. These assays although useful for comparing antioxidant capacities of a variety of chemical or biological samples, do not give information about the antioxidant reactions that may occur in in vitro or in vivo cancer models.
Green tea polyphenols have been shown to act as antioxidants in in vitro models of cancer. Jurkat T cells subjected to oxidative damage by addition of iron II (Fenton) were protected by a green tea extract (10 mg/mL), containing high amounts of EGCG. The protective effect was attributed to the antioxidant capacity of the extract [40], and likely due to hydrogen donation by green tea polypenols [41]. Green tea extract also prevented H2O2 – induced cell death in bladder cancer and normal urothelium cells [42]; and green tea inhibited levels of NO produced from RAW 264.7 macrophages, with an IC50 of 113 μg/mL, either by scavenging or suppression [43]. EGCG was shown to inhibit lipid peroxidation and damage to ATPases in erythrocytes at levels of 1 to 15 μM [44], and to inhibit hydrogen peroxide formation in ultraviolet light (UV)A irradiated keratinocytes [45]. Similarly, green tea extract was shown to decrease formation of hydrogen peroxide and DNA damage induced by UVB irradiation [46]. DNA damage induced by bleomycin in leukocytes was reduced by co-treatment with EGCG, yet EGCG did not alter DNA repair [47]. These results suggest that EGCG scavenges bleomycin-induced reactive oxygen species. Green tea polyphenols such as EGC and EGCG not only scavenge radicals, but may undergo nucleophilic addition with mutagenic electrophiles such as 2-hydroxyamino-3-methylimidazo[4,5-f]quinoline, a carcinogen derived from cooked meat, and thus detoxifying these compounds [48].
Redox sensitive transcription factors, which control cell proliferation and survival, are also affected by green tea polyphenols. EGCG appeared to activate transcription factors nuclear factor (NF)-κκ and activator protein (AP)-1 at low micromolar concentrations [36, 49]; in other studies EGCG also inhibited NF-κκ and AP-1 at these concentrations [50, 51]. These differences could be due to media composition or sensitivity of the cells. EGCG dose-dependently inhibited NF-κκ at higher concentrations of 50 to 300 μM [36]. EC, by contrast, activated NF-κκ, AP-1, and nuclear factor (erythroid-derived 2)-like (Nrf)2 at a concentration of 10 μM [52]. The effects of green tea polyphenols on transcription factors appear to be cell specific and concentration dependent, and the involvement of oxidative mechanisms remains to be clarified.
2.2 In vivo effects
Green tea polyphenols have been reported to have antioxidant effects in vivo. A 4 % increase in human plasma antioxidant capacity, as measured by the FRAP assay, was found 40 min after drinking 400 mL of green tea, and peak FRAP values were found in urine samples after 1 h [53]. The antioxidant capacity of plasma, measured by the Trolox Equivalent Antioxidant Capacity (TEAC) assay, after consuming 150, 300, and 450 mL of green tea (2.5, 5.0, 7.5 g of dried green tea leaves, respectively) increased in a dose dependent fashion [54]. Enhanced plasma antioxidant capacity by 15.6 %, measured by a fluorescence based assay, was found in humans that consume a green tea extract in meat (18.6 mg/d), and the effect was greatest in smokers. Interestingly, the antioxidant capacity in the plasma of subjects returned to baseline after returning to a normal diet [55].
Antioxidant capacity of human plasma, measured by the TEAC assay, was enhanced to a greater extent (1.4 %) by consumption of green tea polyphenols (461.9 mg/d) in tablet form compared to a green tea beverage (697.1 mg/d) [56], suggesting tablets may be an effective way to enhance plasma antioxidant capacity by green tea polyphenols. This difference may be due to pharmacokinetic differences in delivery of polyphenols by tablet compared to beverage. Polyphenols in beverage may be ingested and absorbed more slowly than in tablet. The elevation in antioxidant power of human plasma could be the basis for a role of green tea in cancer preventive effects, yet the effects are likely to be due mainly to metabolites of green tea polyphenols [57–60].
In addition to increasing plasma antioxidant activity, green tea polyphenols can also suppress markers of oxidative stress in vivo. Green tea consumption (4 cups/d for 4 mo) significantly reduced urinary levels of 8-hydroxydeoxyguanosine (8-OHdG), a marker of oxidative stress, by 31 % in a group of smokers [61], particularly in smokers that possessed the active polymorphism for glutathione-S-transferase [62]. Rats that were fed green tea (100 mg/kg B.W.) for 10 days were protected against paracetamol-induced elevation of serum malondialdehyde and catalase and depletion of vitamin C [63]. Rats fed a green tea polyphenol extract equivalent to a human dose of 500 mL of green tea/day for 5 days had less DNA damage in lymphocytes, colonocytes, and hepatocytes due to inherent oxidative stress compared to control rats [64]. In the pancreas of hamsters, consumption of green tea polyphenols (0.1 % in drinking water) inhibited lipid peroxidation and oxidative DNA damage induced by N-nitrosobis(2-oxopropyl)amine [65]. A reduction of leiomyoma fibroid tumors in Quail oviduct by EGCG was accompanied by decreases in liver and serum malondialdehyde (an indication of oxidative stress) and tumor necrosis factor-α (an inflammatory mediator) [66].
Green tea polyphenols also inhibited markers of oxidative stress that are secondary to an inflammatory response. Green tea polyphenols inhibited neutrophil mediated inflammation induced by topical application of 12-O-tetradecanoylphorbol-13-acetate, in the ears of SENCAR mice [67]. Topical application of 1 mg/cm2 EGCG inhibited infiltration of leukocytes and markers of oxidative stress (H2O2 and NO) in the skin of UVB-treated C3H/HeN mice [68]. In addition, a topical application of EGCG on UVB irradiated human skin prevented infiltration of macrophages and neutrophils [69].
3 Direct pro-oxidant effects of green tea
3.1 In vitro effects
Green tea polyphenols accelerate pro-oxidant reactions depending on experimental conditions. EPR has shown that all green tea polyphenols can undergo auto-oxidation at alkaline (pH 13) conditions, which leads to oxidation of the B ring [35]. Similar oxidative reactions have also been shown to occur at physiological pH (7.4) [70]. When EGCG and EGC are reacted with H2O2, the A ring of both compounds become oxidized followed by decarboxylation to form two oxidation products of EGCG and one oxidation product of EGC [71]. This is a pro-oxidant effect as such reactions produce the hydroxyl radical in the presence of iron or copper (Fenton).
The catechol group of green tea polyphenols accelerates ROS production under Fenton conditions (presence of iron II) [72]. Copper II also provides Fenton conditions, and these reactions are perpetuated by the reduction of copper II to copper I by green tea polyphenols, forming the hydroxyl radical and superoxide anion and inducing cellular DNA damage [73][9]. Similar reactions can occur in animal models due to the presence of iron and copper in vivo. For example, copper and iron were detected in normal human tissue, yet the levels were significantly higher in tumors [74]. The complexity of in vivo oxidative mechanisms, however, demands further investigation.
Inhibition of cancer cell viability and induction of apoptosis by green tea polyphenols in vitro appear to be, in part, due to the generation of hydrogen peroxide and superoxide anion [75–77]. One of these studies found that both a green tea extract and EGCG induced apoptosis in HL60 and RAW 264.7 cells, and that in general tea extract was more effective. The genotoxicity (measured by the comet assay) of 10 μM EGCG was partially explained by the production of hydrogen peroxide. The remaining activity could be due to production of superoxide radical [75]. This finding was strengthened by another study that reported partially reduced activity, measured by reduction of cell viability and markers of oxidative stress, when EGCG was co-treated with superoxide dismutase and catalase [76]. On the contrary, addition of superoxide dismutase to EGCG treated esophageal squamous cancer cells increased the inhibitory effect by stabilizing EGCG [78]. It may be that the target-specific effects of EGCG are more important than pro-oxidant effects for inhibiting growth of these cells, although such a target is unknown. Production of hydrogen peroxide in cancer cells by green tea polyphenols has also been measured directly [49]. ECG, EGCG, and EGC have been shown to inhibit viability of prostate cancer cells by inducing apoptosis, an effect attributed to ROS (H2O2 and superoxide anion) formation. EC was not active under these conditions [79]. The inhibition of both HeLa and lymphoblastoid cell lines appeared to be due to the pro-oxidant effect of EGCG [15, 80]. The production of hydrogen peroxide and superoxide anion in vitro occurs mostly in the media, yet these reactive oxygen species (ROS) may also be formed intracellularly [16]. In the former case, ROS can be eliminated by addition of exogenous SOD/catalase. In the latter, intracellular ROS are not accessible to SOD/catalase. Inhibition of intracellular ROS would require addition of a soluble antioxidant such as N-acetylcysteine (NAC). Li et al. found that addition of NAC had such an effect in human lung cancer cells [76].
Redox sensitive markers of inflammation and survival are also affected by green tea polyphenols in vitro. Low levels of hydrogen peroxide (10 μM) in HT-29 colon cancer cells induced expression of cyclooxygenase-2, yet this effect was suppressed and accompanied by induction of apoptosis and inhibition of cell growth at higher concentrations (100 μM) [81]. A gene profiling study using Ha-ras transformed bronchial epithelial cells found that EGCG activation of specific groups of apoptosis-related genes was mediated by hydrogen peroxide, whereas expression of another set of apoptosis-related genes was hydrogen peroxide independent [82]. The inhibitory properties of EGCG appear therefore to be due to both ROS-dependent and independent mechanisms. The relative importance of each needs to be further assessed both in vitro and in vivo. EGCG has been shown to activate matrix metalloproteinase (MMP)-7 (an AP-1 regulated protein) in HT-29 cells at a concentration of 25 M, however in other studies, EGCG has been shown to inhibit MMPs [49]. most likely by inducing oxidative stress. This differential effect may be cell and concentration dependent. EGCG has also induced apoptosis and caused growth arrest in pancreatic cancer cells, due to activation of c-Jun N-terminal kinase signaling by ROS [83].
Nrf2 appears to play an important role in protecting cancer cells from green tea polyphenol mediated oxidative stress. A549 human lung cancer cells were resistant to EGCG induced apoptosis. This cell line overexpresses Nrf2, protecting it from ROS-mediated apoptosis; yet high concentrations (> 200 μM) of EGCG suppressed Nrf2, resulting in apoptosis [84]. Nrf2 could be an important target for the prevention of cancer, as inhibition of Nrf2 causes cancer cells to be more susceptible to ROS-mediated cell death.
Concentration is a factor that could determine whether green tea polyphenols act as antioxidants or pro-oxidants in vitro. EGC and EGCG, both generate hydrogen peroxide at concentrations greater than 10 μM [85]. This was shown in lymphoblastoid cell lines, where both EGCG and ascorbic acid at levels of 1 to 10 μM offered DNA protection against bleomycin, yet the protective effects were lost at 100 μM [86]. Green tea polyphenols do not appear to induce ROS-mediated DNA damage below concentrations of 10 μM, but rather prevent hydrogen peroxide mediated DNA damage in a dose dependent manner at lower concentrations [85]. In general, concentrations of green tea polypheols in excess of 10 μM upset redox balance when added to cells, creating a pro-oxidant environment. This may also be true in vivo, as the total concentration of green tea polyphenols can exceed 10 μM in human biological samples [87].
EGCG can also covalently bind to and inhibit various proteins through oxidative mechanisms. It can covalently bind to the cysteinyl thiol residues of proteins through an autooxidation mechanism, thereby inhibiting those proteins [88]. Specifically, quinones can be formed from EGCG B or D rings, which then covalently react with nucleophilic thiols on proteins. This autooxidation mechanism is facilitated by an increase in pH. These reactions can be prevented by the addition of SOD, indicating that the superoxide radical is part of the autooxidation mechanism. The process can be slowed with the addition of glutathione, indicating that inhibition of proteins by this mechanism may rely on redox status [88]. It is also possible that glutathione competes for protein binding or that EGCG forms thiol conjugates with glutathione. The latter was shown by Sang et al., where oxidized EGCG formed thiol conjugates with cysteine or glutathione, and these conjugates were present in mouse urine [89].
3.2 In vivo effects
Evidence also exists for a potential pro-oxidant basis for the anti-cancer effects of tea polyphenols in vivo. H2O2 was generated in the oral cavity of human subjects by either holding green tea in the mouth or chewing green tea leaves. The production of H2O2 was directly proportional to the concentration of green tea polyphenols in the mouth, and has implications for oral cancer prevention [90]. Dietary EGCG reduced tumor growth in a xenograft mouse model of lung cancer dose-dependently. Inhibition was characterized by cancer cell apoptosis and induction of oxidative damage to tumor cell DNA (8-OHdG), but not in normal tissues [76]. Decaffeinated green tea (0.6 % in water) reduced genitourinary tumor load in rats, concurrent with formation of 8-OHdG and 4-hydroxynonenal, both indicators of oxidative damage [91].
Although the anti-cancer effects of EGCG may be attributed to a pro-oxidant effect, high oral doses of EGCG (750 – 1500 mg/kg) have been shown to exert hepatotoxic effects in CF-1 mice. The hepatotoxic effects were associated with increased markers of oxidative stress such as lipid peroxidation, plasma 8-isoprostane, metallothionein, and γ-histone 2AX protein [92]. Therefore dose is an important factor in cancer preventive effects of green tea polyphenols, and the role of pro-oxidant effects at different doses must be carefully assessed.
4. Indirect antioxidant effects of green tea
Green tea polyphenols can have indirect antioxidant effects in vivo. Intraperitoneal injections with green tea polyphenols, have been shown to increase levels of phase II antioxidant enzymes in rat livers including glutathione peroxidase and reductase, glutathione-S-transferase (GST), catalase, quinone reductase, and superoxide dismutase [72]. This highlights the complexity of in vivo oxidative mechanisms, as phase II antioxidant enzymes respond to oxidative stress induced by polyphenols in order to restore balance.
Green tea polyphenols have also been shown to increase levels of phase II antioxidant enzymes in the small intestine, lungs, and skin of mice [93], the prostate of rats [91], and the oral cavity of hamsters [94]. This may be mediated through activation of MAPKs by green tea polyphenols [95]. Alternatively, Nrf2 signaling may play a role. Cecum tumor reduction in EGCG fed mice was associated with increased levels of Nrf2 protein and gene expression [96]. In C57BL/6J mice, oral treatment with EGCG (200 mg/kg for 3 or 12 h) caused both induction and suppression of expression of numerous genes in the liver and small intestine, importantly Nrf2-regulated phase II genes such as heme oxygenase 1 and alcohol dehydrogenase were induced [97].
Activity of GST was elevated in humans after consuming 800 mg EGCG per day (in polyphenon E tablets) for 4 weeks. The effect, however, was only observed in subjects with low GST baseline activity. Activity of GST was actually decreased in subjects with high baseline activity [98]. These results suggest that production of ROS by green tea polyphenols occurs in subjects with low baseline oxidative stress, whereas green tea polyphenols may be scavenging ROS in subjects with high baseline oxidative stress (measured as GST activity).
Green tea containing tannins protected rats against arsenic-induced oxidative stress by increasing levels of reduced glutathione, superoxide dismutase, and glutathione peroxidase. This up-regulation of phase II antioxidant defense was concurrent with less hepatic damage and inflammation. Tannin rich green tea increased phase II antioxidant defense better than de-tannified green tea (same weight basis) [99]. This indicates that the indirect antioxidant activity of green tea is due, in part, to tannin content.
Activation of phase II enzymes is important, not only for scavenging of ROS, but also for detoxification of carcinogens such as aflatoxin B1. Aflatoxin B1 was better excreted into urine of human subjects that consumed 500 and 1000 mg/d of green tea polyphenols for 3 mo, through phase II conjugation with NAC [100].
5. Summary and conclusions
In vitro antioxidant assays provide a quick way to compare antioxidant activities of different samples, yet they have questionable relevance to disease prevention in vivo. Green tea polyphenols, or their metabolites, present in biological samples act as antioxidants in many of these assays, yet their mechanisms of action in vivo may differ depending on physiological redox status, proposed tissue site of action, and other factors (or through a non-antioxidant related mechanism).
Green tea polyphenols may act as either antioxidants or pro-oxidants to exert protective effects against cancer (Fig 3). Studies have shown that consumption of green tea can either induce oxidative stress, leading to ROS-mediated cancer cell death, or they can scavenge ROS under conditions of high oxidative stress, preventing cellular damage. Transcription factors such as NF-κκ and AP-1 regulate cancer cell survival and proliferation and may be modulated in part by ROS. Green tea polyphenols have been shown to affect these factors in varying ways depending on the dose of polyphenols and physiologic context of the interaction. Green tea polyphenols are, therefore, compounds that appear to protect against cancer under various physiological conditions, and may be viewed as providing redox balance in the context of disease.
Figure 3.
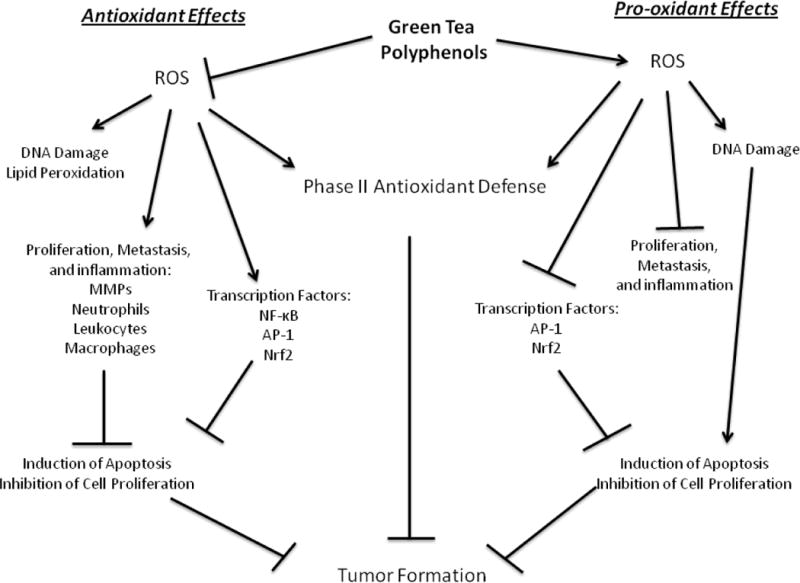
Propose antioxidant and pro-oxidant effects of green tea polyphenols relevant to the prevention of cancer
Concentration, structure, cell type, and experimental conditions (including pH and redox status) are therefore important when considering if green tea inhibits growth of cancer cells through antioxidant or pro-oxidant mechanisms. In vitro studies have provided an understanding of how green tea polyphenols act as pro-oxidants, yet more in vivo work is required. Real-time imaging for detection of ROS in animal cancer models [101] and EPR measurements of biological samples are methods that should be utilized in the future to better understand the antioxidant or pro-oxidant mechanisms of green tea polyphenols in cancer prevention. Continued mechanism-based studies in human stubjects are also needed to determine the relevance of putative antioxidant or pro-oxidant effects for cancer prevention in humans.
Acknowledgments
Supported by National Institutes of Health Grant AT004678.
Glossary
Abbreviations
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- AP
activator protein
- EC
(–)-epicatechin
- ECG
(–)-epicatechin-3-gallate
- EGC
(–)-epigallocatechin
- EGCG
(–)-epigallocatechin-3-gallate
- FRAP
ferric reducing/antioxidant power, FRAP
- NFκB
nuclear factor kappa B
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- ORAC
oxygen radical absorbance capacity
- TEAC
TROLOX equivalent antioxidant capacity
Footnotes
Conflict of Interest
Neither author has a conflict of interest to disclose.
References
- 1.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukushima Y, Ohie T, Yonekawa Y, Yonemoto K, Aizawa H, Mori Y, Watanabe M, Takeuchi M, Hasegawa M, Taguchi C, Kondo K. Coffee and Green Tea As a Large Source of Antioxidant Polyphenols in the Japanese Population. J Agric Food Chem. 2009;57:1253–1259. doi: 10.1021/jf802418j. [DOI] [PubMed] [Google Scholar]
- 3.Baptista JAB, Tavares JFD, Carvalho RCB. Comparison of catechins and aromas among different green teas using HPLC/SPME-GC. Food Res Int. 1998;31:729–736. [Google Scholar]
- 4.Shishikura Y, Khokhar S. Factors affecting the levels of catechins and caffeine in tea beverage: estimated daily intakes and antioxidant activity. J Sci Food Agric. 2005;85:2125–2133. [Google Scholar]
- 5.Engelhardt UH, Lakenbrink C, Pokorny O. In: Nutraceutical Beverages: Chemistry, Nutrition, and Health Effects. Shahidi F, Weerasinghe DK, editors. Amer Chemical Soc; Washington: 2004. pp. 254–264. [Google Scholar]
- 6.Lin YS, Tsai YJ, Tsay JS, Lin JK. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J Agric Food Chem. 2003;51:1864–1873. doi: 10.1021/jf021066b. [DOI] [PubMed] [Google Scholar]
- 7.Lin SD, Liang CH, Liu EH, Mau JL. ANTIOXIDANT PROPERTIES OF WATER EXTRACTS FROM PARCHING GREEN TEA. J Food Biochem. 2010;34:477–500. [Google Scholar]
- 8.Long LH, Lan ANB, Hsuan FTY, Halliwell B. Generation of hydrogen peroxide by "antioxidant" beverages and the effect of milk addition. Is cocoa the best beverage? Free Radic Res. 1999;31:67–71. doi: 10.1080/10715769900300611. [DOI] [PubMed] [Google Scholar]
- 9.Azam S, Hadi N, Khan NU, Hadi SM. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol Vitro. 2004;18:555–561. doi: 10.1016/j.tiv.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Cui Y, Morgenstern H, Greenland S, Tashkin DP, Mao JT, Cai L, Cozen W, Mack TM, Lu QY, Zhang ZF. Dietary flavonoid intake and lung cancer - A population-based case-control study. Cancer. 2008;112:2241–2248. doi: 10.1002/cncr.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan JM, Koh WP, Sun CL, Lee HP, Yu MC. Green tea intake, ACE gene polymorphism and breast cancer risk among Chinese women in Singapore. Carcinogenesis. 2005;26:1389–1394. doi: 10.1093/carcin/bgi080. [DOI] [PubMed] [Google Scholar]
- 12.Yu GP, Hsieh CC, Wang LY, Yu SZ, Li XL, Jin TH. GREEN-TEA CONSUMPTION AND RISK OF STOMACH-CANCER - A POPULATION-BASED CASE-CONTROL STUDY IN SHANGHAI, CHINA. Cancer Causes Control. 1995;6:532–538. doi: 10.1007/BF00054162. [DOI] [PubMed] [Google Scholar]
- 13.Nagle CM, Olsen CM, Bain CJ, Whiteman DC, Green AC, Webb PM. Tea consumption and risk of ovarian cancer. Cancer Causes Control. 2010;21:1485–1491. doi: 10.1007/s10552-010-9577-7. [DOI] [PubMed] [Google Scholar]
- 14.Ji SJ, Han DH, Kim JH. Inhibition of proliferation and induction of apoptosis by EGCG in human osteogenic sarcoma (HOS) cells. Arch Pharm Res. 2006;29:363–368. doi: 10.1007/BF02968585. [DOI] [PubMed] [Google Scholar]
- 15.Noda C, He J, Takano T, Tanaka C, Kondo T, Tohyama K, Yamamura H, Tohyama Y. Induction of apoptosis by epigallocatechin-3-gallate in human lymphoblastoid B cells. Biochem Biophys Res Commun. 2007;362:951–957. doi: 10.1016/j.bbrc.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 16.Nakazato T, Ito K, Miyakawa Y, Kinjo K, Hozumi N, Ikeda Y, Kizaki M. Catechin, a green tea component, rapidly induces apoptosis of myeloid leukemic cells via modulation of reactive oxygen species production in vitro and inhibits tumor growth in vivo. Haematologica. 2005;90:317–325. [PubMed] [Google Scholar]
- 17.Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (–)-epigallocatechin-3-gallate on human melanoma: Possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114:513–521. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- 18.Li HC, Yashiki S, Sonoda J, Lou H, Ghosh SK, Byrnes JJ, Lema C, Fujiyoshi T, Karasuyama M, Sonoda S. Green tea polyphenols induce apoptosis in vitro in peripheral blood T lymphocytes of adult T-cell leukemia patients. Jpn J Cancer Res. 2000;91:34–40. doi: 10.1111/j.1349-7006.2000.tb00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JH, Jeong YJ, Lee SW, Kim D, Oh SJ, Lim HS, Oh HK, Kim SH, Kim WJ, Jung JY. EGCG induces apoptosis in human laryngeal epidermoid carcinoma Hep2 cells via mitochondria with the release of apoptosis-inducing factor and endonuclease G. Cancer Lett. 2010;290:68–75. doi: 10.1016/j.canlet.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 20.Vergote D, Cren-Olive C, Chopin V, Toillon RA, Rolando C, Hondermarck H, Le Bourhis XF. (–)-epigallocatechin (EGC) of green tea induces apoptosis of human breast cancer cells but not of their normal counterparts. Breast Cancer Res Treat. 2002;76:195–201. doi: 10.1023/a:1020833410523. [DOI] [PubMed] [Google Scholar]
- 21.Kavanagh KT, Hafer LJ, Kim DW, Mann KK, Sherr DH, Rogers AE, Sonenshein GE. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J Cell Biochem. 2001;82:387–398. doi: 10.1002/jcb.1164. [DOI] [PubMed] [Google Scholar]
- 22.Lin JK, Liang YC, Lin-Shiau SY. Cancer chemoprevention by tea polyphenols through mitotic signal transduction blockade. Biochem Pharmacol. 1999;58:911–915. doi: 10.1016/s0006-2952(99)00112-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhou B, Pan J, Dai F, Zhao CY, Zhang LP, Wei QY, Yang L, Zheng RL, Liu ZL. Redifferentiation of human hepatoma cells induced by green tea polyphenols. Res Chem Intermed. 2004;30:627–636. [Google Scholar]
- 24.Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, Asakage M, Sasaki S, Watanabe T, Takahashi K, Nagawa H. Epigallocatechin gallate induces apoptosis of monocytes. J Allergy Clin Immunol. 2005;115:186–191. doi: 10.1016/j.jaci.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Kuroiwa Y, Ishii Y, Umemura T, Kanki K, Mitsumori K, Nishikawa A, Nakazawa H, Hirose M. Combined treatment with green tea catechins and sodium nitrite selectively promotes rat forestomach carcinogenesis after initiation with N-methyl-N′-nitro-N-nitrosoguanidine. Cancer Sci. 2007;98:949–957. doi: 10.1111/j.1349-7006.2007.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valcic S, Timmermann BN, Alberts DS, Wachter GA, Krutzsch M, Wymer J, Guillen JM. Inhibitory effect of six green tea catechins and caffeine on the growth of four selected human tumor cell lines. Anti-Cancer Drugs. 1996:7, 461–468. doi: 10.1097/00001813-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Lin YL, Juan IM, Chen YL, Liang YC, Lin JK. Composition of polyphenols in fresh tea leaves and associations of their oxygen-radical-absorbing capacity with antiproliferative actions in fibroblast cells. J Agric Food Chem. 1996;44:1387–1394. [Google Scholar]
- 28.Yamamoto T, Hsu S, Lewis J, Wataha J, Dickinson D, Singh B, Bollag WB, Lockwood P, Ueta E, Osaki T, Schuster G. Green tea polyphenol causes differential oxidative environments in tumor versus normal epithelial cells. J Pharmacol Exp Ther. 2003;307:230–236. doi: 10.1124/jpet.103.054676. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Wang FF, Yu F, Fang Y, Xin ZH, Yang FM, Xu J, Zhao LY, Hu QH. In vitro antioxidant and anticancer activities of ethanolic extract of selenium-enriched green tea. Food Chem. 2008;111:165–170. [Google Scholar]
- 30.Li HJ, Li F, Yang FM, Fang Y, Xin ZH, Zhao LY, Hu QH. Size effect of Se-enriched green tea particles on in vitro antioxidant and antitumor activities. J Agric Food Chem. 2008;56:4529–4533. doi: 10.1021/jf0731200. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Zhu SG, Yang FM, Cheng LC, Hu Y, Pan GX, Hu QH. The influence of selenium on the antioxidant activity of green tea. J Sci Food Agric. 2003;83:451–455. [Google Scholar]
- 32.Xu J, Yang FM, An XX, Hu QH. Anticarcinogenic activity of selenium-enriched green tea extracts in vivo. J Agric Food Chem. 2007;55:5349–5353. doi: 10.1021/jf070568s. [DOI] [PubMed] [Google Scholar]
- 33.Sukhthankar M, Yamaguchi K, Lee SH, McEntee MF, Eling TE, Hara Y, Baek SJ. A green tea component suppresses posttranslational expression of basic fibroblast growth factor in colorectal cancer. Gastroenterology. 2008;134:1972–1980. doi: 10.1053/j.gastro.2008.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ran ZH, Chen C, Xiao SD. Epigallocatechin-3–gallate ameliorates rats colitis induced by acetic acid. Biomed Pharmacother. 2008;62:189–196. doi: 10.1016/j.biopha.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Severino JF, Goodman BA, Kay CWM, Stolze K, Tunega D, Reichenauer TG, Pirker KF. Free radicals generated during oxidation of green tea polyphenols: Electron paramagnetic resonance spectroscopy combined with density functional theory calculations. Free Radic Biol Med. 2009;46:1076–1088. doi: 10.1016/j.freeradbiomed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Shi XL, Ye JP, Leonard SS, Ding M, Vallyathan V, Castranova V, Rojanasakul Y, Dong ZG. Antioxidant properties of (–)-epicatechin-3-gallate and its inhibition of Cr(VI)-induced DNA damage and Cr(IV)- or TPA-stimulated NF-kappa B activation. Mol Cell Biochem. 2000;206:125–132. doi: 10.1023/a:1007012403691. [DOI] [PubMed] [Google Scholar]
- 37.Benzie IFF, Szeto YT. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J Agric Food Chem. 1999;47:633–636. doi: 10.1021/jf9807768. [DOI] [PubMed] [Google Scholar]
- 38.Chandra S, de Mejia EG. Polyphenolic compounds, antioxidant capacity, and quinone reductase activity of an aqueous extract of Ardisia compressa in comparison to mate (Ilex paraguariensis) and green (Camellia sinensis) teas. J Agric Food Chem. 2004;52:3583–3589. doi: 10.1021/jf0352632. [DOI] [PubMed] [Google Scholar]
- 39.Henning SM, Fajardo-Lira C, Lee HW, Youssefian AA, Go VLW, Heber D. Catechin content of 18 teas and a green tea extract supplement correlates with the antioxidant capacity. Nutr Cancer. 2003;45:226–235. doi: 10.1207/S15327914NC4502_13. [DOI] [PubMed] [Google Scholar]
- 40.Erba D, Riso P, Colombo A, Testolin G. Supplementation of Jurkat T cells with green tea extract decreases oxidative damage due to iron treatment. J Nutr. 1999;129:2130–2134. doi: 10.1093/jn/129.12.2130. [DOI] [PubMed] [Google Scholar]
- 41.Anderson RF, Fisher LJ, Hara Y, Harris T, Mak WB, Melton LD, Packer JE. Green tea catechins partially protect DNA from (.)OH radical-induced strand breaks and base damage through fast chemical repair of DNA radicals. Carcinogenesis. 2001;22:1189–1193. doi: 10.1093/carcin/22.8.1189. [DOI] [PubMed] [Google Scholar]
- 42.Coyle CH, Philips BJ, Morrisroe SN, Chancellor MB, Yoshimura N. Antioxidant effects of green tea and its polyphenols on bladder cells. Life Sci. 2008;83:12–18. doi: 10.1016/j.lfs.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai PJ, Tsai TH, Yu CH, Ho SC. Comparison of NO-scavenging and NO-suppressing activities of different herbal teas with those of green tea. Food Chem. 2007;103:181–187. [Google Scholar]
- 44.Saffari Y, Sadrzadeh SMH. Green tea metabolite EGCG protects membranes against oxidative damage in vitro. Life Sci. 2004;74:1513–1518. doi: 10.1016/j.lfs.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Huang CC, Fang JY, Wu WB, Chiang HS, Wei YJ, Hung CF. Protective effects of (–)-epicatechin-3-gallate on UVA-induced damage in HaCaT keratinocytes. Arch Dermatol Res. 2005;296:473–481. doi: 10.1007/s00403-005-0540-5. [DOI] [PubMed] [Google Scholar]
- 46.Wei HC, Zhang XS, Zhao JF, Wang ZY, Bickers D, Lebwohl M. Scavenging of hydrogen peroxide and inhibition of ultraviolet light-induced oxidative DNA damage by aqueous extracts from green and black teas. Free Radic Biol Med. 1999;26:1427–1435. doi: 10.1016/s0891-5849(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 47.Glei M, Pool-Zobel BL. The main catechin of green tea, (–)-epigallocatechin-3-gallate (EGCG), reduces bleomycin-induced DNA damage in human leucocytes. Toxicol Vitro. 2006;20:295–300. doi: 10.1016/j.tiv.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Hernaez JF, Xu MR, Dashwood RH. Antimutagenic activity of tea towards 2-hydroxyamino-3-methylimidazo 4,5-f quinoline: effect of tea concentration and brew time on electrophile scavenging. Mutat Res.-Fundam Mol Mech Mutagen. 1998;402:299–306. doi: 10.1016/s0027-5107(97)00309-6. [DOI] [PubMed] [Google Scholar]
- 49.Kim M, Murakami A, Ohigashi H. Modifying effects of dietary factors on (–)-epigallocatechin-3-gallate-induced pro-matrix metalloproteinase-7 production in HT-29 human colorectal cancer cells. Biosci Biotechnol Biochem. 2007;71:2442–2450. doi: 10.1271/bbb.70213. [DOI] [PubMed] [Google Scholar]
- 50.Barthelman M, Bair WB, Stickland KK, Chen WX, Timmermann N, Valcic S, Dong ZG, Bowden GT. (–)-epigallocatechin-3-gallate inhibition of ultraviolet B induced AP-1 activity. Carcinogenesis. 1998;19:2201–2204. doi: 10.1093/carcin/19.12.2201. [DOI] [PubMed] [Google Scholar]
- 51.Yang JY, Wei DZ, Liu JW. Repressions of MMP-9 expression and NF-kappa B localization are involved in inhibition of lung carcinoma 95-D cell invasion by (–)-epigallocatechin-3-gallate. Biomed Pharmacother. 2005;59:98–103. doi: 10.1016/j.biopha.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Granado-Serrano AB, Martin MA, Haegeman G, Goya L, Bravo L, Ramos S. Epicatechin induces NF-kappa B, activator rotein-1 (AP-1) and nuclear transcription factor erythroid 2p45-related factor-2 (Nrf2) via phosphatidylinositol-3-kinase/protein kinase B (PI3K/AKT) and extracellular regulated kinase (ERK) signalling in HepG2 cells. Br J Nutr. 2010;103:168–179. doi: 10.1017/S0007114509991747. [DOI] [PubMed] [Google Scholar]
- 53.Benzie IFF, Szeto YT, Strain JJ, Tomlinson B. Consumption of green tea causes rapid increase in plasma antioxidant power in humans. Nutr Cancer. 1999;34:83–87. doi: 10.1207/S15327914NC340112. [DOI] [PubMed] [Google Scholar]
- 54.Sung H, Nah J, Chun S, Park H, Yang SE, Min WK. In vivo antioxidant effect of green tea. Eur J Clin Nutr. 2000;54:527–529. doi: 10.1038/sj.ejcn.1600994. [DOI] [PubMed] [Google Scholar]
- 55.Young JF, Dragsted LO, Haraldsdottir J, Daneshvar B, Kall MA, Loft S, Nilsson L, Nielsen SE, Mayer B, Skibsted LH, Huynh-Ba T, Hermetter A, Sandstrom B. Green tea extract only affects markers of oxidative status postprandially: lasting antioxidant effect of flavonoid-free diet. Br J Nutr. 2002;87:343–355. doi: 10.1079/bjnbjn2002523. [DOI] [PubMed] [Google Scholar]
- 56.Henning SM, Niu YT, Lee NH, Thames GD, Minutti RR, Wang HJ, Go VLW, Heber D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am J Clin Nutr. 2004;80:1558–1564. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, Zheng Y, Chow MSS, Zuo Z. Investigation of intestinal absorption and disposition of green tea catechins by Caco-2 monolayer model. Int J Pharm. 2004;287:1–12. doi: 10.1016/j.ijpharm.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 58.Takagaki A, Nanjo F. Metabolism of (–)-Epigallocatechin Gallate by Rat Intestinal Flora. J Agric Food Chem. 2010;58:1313–1321. doi: 10.1021/jf903375s. [DOI] [PubMed] [Google Scholar]
- 59.van’t Slot G, Humpf HU. Degradation and Metabolism of Catechin, Epigallocatechin-3-gallate (EGCG), and Related Compounds by the Intestinal Microbiota in the Pig Cecum Model. J Agric Food Chem. 2009;57:8041–8048. doi: 10.1021/jf900458e. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, Chow MSS, Zuo Z. Effect of the co-occurring components from green tea on the intestinal absorption and disposition of green tea polyphenols in Caco-2 monolayer model. J Pharm Pharmacol. 2006;58:37–44. doi: 10.1211/jpp.58.1.0005. [DOI] [PubMed] [Google Scholar]
- 61.Hakim IA, Harris RB, Brown S, Chow HHS, Wiseman S, Agarwal S, Talbot W. Effect of increased tea consumption on oxidative DNA damage among smokers: A randomized controlled study. J Nutr. 2003;133:3303S–3309S. doi: 10.1093/jn/133.10.3303S. [DOI] [PubMed] [Google Scholar]
- 62.Hakim IA, Harris RB, Chow HHS, Dean M, Brown S, Ali IU. Effect of a 4-month tea intervention on oxidative DNA damage among heavy smokers: Role of glutathione S-transferase genotypes. Cancer Epidemiol Biomarkers Prev. 2004;13:242–249. doi: 10.1158/1055-9965.epi-03-0193. [DOI] [PubMed] [Google Scholar]
- 63.Ojo OO, Kabutu FR, Bello M, Babayo U. Inhibition of paracetamol-induced oxidative stress in rats by extracts of lemongrass (Cymbropogon citratus) and green tea (Camellia sinensis) in rats. Afr J Biotechnol. 2006:5, 1227–1232. [Google Scholar]
- 64.Kager N, Ferk F, Kundi M, Wagner KH, Misik M, Knasmueller S. Prevention of oxidative DNA damage in inner organs and lymphocytes of rats by green tea extract. European Journal of Nutrition. 2010;49:227–234. doi: 10.1007/s00394-009-0068-0. [DOI] [PubMed] [Google Scholar]
- 65.Takabayashi F, Harada N, Tahara S, Kaneko T, Hara Y. Effect of green tea catechins on the amount of 8-hydroxydeoxyguanosine (8-OHdG) in pancreatic and hepatic DNA after a single administration of N-nitrosobis(2-oxopropyl)amine (BOP) Pancreas. 1997;15:109–112. doi: 10.1097/00006676-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Ozercan IH, Sahin N, Akdemir F, Onderci M, Seren S, Sahin K, Kucuk O. Chemoprevention of fibroid tumors by - -epigallocatechin-3-gallate in quail. Nutr Res. 2008;28:92–97. doi: 10.1016/j.nutres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 67.Katiyar SK, Agarwal R, Ekker S, Wood GS, Mukhtar H. PROTECTION AGAINST 12-O-TETRADECANOYLPHORBOL-13-ACETATE-CAUSED INFLAMMATION IN SENCAR MOUSE EAR SKIN BY POLYPHENOLIC FRACTION ISOLATED FROM GREEN TEA. Carcinogenesis. 1993;14:361–365. doi: 10.1093/carcin/14.3.361. [DOI] [PubMed] [Google Scholar]
- 68.Katiyar SK, Mukhtar H. Green tea polyphenol (–)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J Leukoc Biol. 2001;69:719–726. [PubMed] [Google Scholar]
- 69.Katiyar SK, Matsui MS, Elmets CA, Mukhtar H. Polyphenolic antioxidant (–)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol. 1999;69:148–153. [PubMed] [Google Scholar]
- 70.Sang SM, Lee MJ, Hou Z, Ho CT, Yang CS. Stability of tea polyphenol (–)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J Agric Food Chem. 2005;53:9478–9484. doi: 10.1021/jf0519055. [DOI] [PubMed] [Google Scholar]
- 71.Zhu NQ, Huang TC, Yu YN, LaVoie EJ, Yang CS, Ho CT. Identification of oxidation products of (–)-epigallocatechin gallate and (–)-epigallocatechin with H2O2. J Agric Food Chem. 2000;48:979–981. doi: 10.1021/jf991188c. [DOI] [PubMed] [Google Scholar]
- 72.Lee SF, Liang YC, Lin JK. Inhibition of 1,2,4-benzenetriol-generated active oxygen species and induction of phase II enzymes by green tea polyphenols. Chem.-Biol Interact. 1995;98:283–301. doi: 10.1016/0009-2797(95)03652-0. [DOI] [PubMed] [Google Scholar]
- 73.Malik A, Azam S, Hadi N, Hadi SM. DNA degradation by water extract of green tea in the presence of copper ions: Implications for anticancer properties. Phytother Res. 2003;17:358–363. doi: 10.1002/ptr.1149. [DOI] [PubMed] [Google Scholar]
- 74.Lavilla I, Costas M, San Miguel P, Millos J, Bendicho C. Elemental fingerprinting of tumorous and adjacent non-tumorous tissues from patients with colorectal cancer using ICP-MS, ICP-OES and chemometric analysis. Biometals. 2009;22:863–875. doi: 10.1007/s10534-009-9231-6. [DOI] [PubMed] [Google Scholar]
- 75.Elbling L, Weiss RM, Teufelhofer O, Uhl M, Knasmueller S, Schulte-Hermann R, Berger W, Mickshe M. Green tea extract and (–)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. Faseb J. 2005;19:807. doi: 10.1096/fj.04-2915fje. [DOI] [PubMed] [Google Scholar]
- 76.Li GX, Chen YK, Hou Z, Xiao H, Jin HY, Lu G, Lee MJ, Liu B, Guan F, Yang ZH, Yu A, Yang CS. Pro-oxidative activities and dose-response relationship of (–)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: a comparative study in vivo and in vitro. Carcinogenesis. 2010;31:902–910. doi: 10.1093/carcin/bgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakazato T, Ito K, Ikeda Y, Kizaki M. Green tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clin Cancer Res. 2005;11:6040–6049. doi: 10.1158/1078-0432.CCR-04-2273. [DOI] [PubMed] [Google Scholar]
- 78.Hou Z, Sang SM, You H, Lee MJ, Hong J, Chin KV, Yang CS. Mechanism of action of (–)-epigallocatechin-3-gallate: Auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- 79.Chung LY, Cheung TC, Kong SK, Fung KP, Choy YM, Chen ZY, Kwok TT. Induction of apoptosis by green tea catechins in human prostate cancer DU145 cells. Life Sci. 2001;68:1207–1214. doi: 10.1016/s0024-3205(00)01020-1. [DOI] [PubMed] [Google Scholar]
- 80.Cutter H, Wu LY, Kim C, Morre DJ, Morre DM. Is the cancer protective effect correlated with growth inhibitions by green tea (–)-epigallocatechin gallate mediated through an antioxidant mechanism? Cancer Lett. 2001;162:149–154. doi: 10.1016/s0304-3835(00)00631-5. [DOI] [PubMed] [Google Scholar]
- 81.Park IJ, Lee YK, Hwang JT, Kwon DY, Ha J, Park OJ. In: Natural Compounds and Their Role in Apoptotic Cell Signaling Pathways. Diederich M, editor. Blackwell Publishing; Oxford: 2009. pp. 538–544. [Google Scholar]
- 82.Vittal R, Selvanayagam ZE, Sun Y, Hong J, Liu F, Chin KV, Yang CS. Gene expression changes induced by green tea polyphenol (–)-epigallocatechin-3-gallate in human bronchial epithelial 21BES cells analyzed by DNA microarray. Mol Cancer Ther. 2004;3:1091–1099. [PubMed] [Google Scholar]
- 83.Qanungo S, Das M, Haldar S, Basu A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis. 2005;26:958–967. doi: 10.1093/carcin/bgi040. [DOI] [PubMed] [Google Scholar]
- 84.Kweon MH, Adhami VM, Lee JS, Mukhtar H. Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J Biol Chem. 2006;281:33761–33772. doi: 10.1074/jbc.M604748200. [DOI] [PubMed] [Google Scholar]
- 85.Sugisawa A, Kimura M, Fenech M, Umegaki K. Anti-genotoxic effects of tea catechins against reactive oxygen species in human lymphoblastoid cells. Mutat Res Genet Toxicol Environ Mutagen. 2004;559:97–103. doi: 10.1016/j.mrgentox.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 86.Hsu TC, Zhao YJ, Wang RY, Wu YF, Spitz MR, Hong WK. Comparative efficacy as antioxidants between ascorbic acid and epigallocatechin gallate on cells of two human lymphoblastoid lines. Cancer Genet Cytogenet. 2001;124:169–171. doi: 10.1016/s0165-4608(00)00337-x. [DOI] [PubMed] [Google Scholar]
- 87.Stalmach A, Troufflard S, Serafini M, Crozier A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol Nutr Food Res. 2009;53:S44–S53. doi: 10.1002/mnfr.200800169. [DOI] [PubMed] [Google Scholar]
- 88.Ishii T, Mori T, Tanaka T, Mizuno D, Yamaji R, Kumazawa S, Nakayama T, Akagawa M. Covalent modification of proteins by green tea polyphenol (–)-epigallocatechin-3-gallate through autoxidation. Free Radic Biol Med. 2008;45:1384–1394. doi: 10.1016/j.freeradbiomed.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 89.Sang SM, Lambert JD, Hong J, Tian SY, Lee MJ, Stark RE, Ho CT, Yang CS. Synthesis and structure identification of thiol conjugates of (–)-epigallocatechin gallate and their urinary levels in mice. Chem Res Toxicol. 2005;18:1762–1769. doi: 10.1021/tx050151l. [DOI] [PubMed] [Google Scholar]
- 90.Lambert JD, Kwon SJ, Hong J, Yang CS. Salivary hydrogen peroxide produced by holding or chewing green tea in the oral cavity. Free Radic Res. 2007;41:850–853. doi: 10.1080/10715760601091659. [DOI] [PubMed] [Google Scholar]
- 91.O’Sullivan J, Sheridan J, Mulcahy H, Tenniswood M, Morrissey C. The effect of green tea on oxidative damage and tumour formation in Lobund-Wistar rats. Eur J Cancer Prev. 2008;17:489–501. doi: 10.1097/CEJ.0b013e3282f0c04e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lambert JD, Kennett MJ, Sang SM, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (–)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48:409–416. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khan SG, Katiyar SK, Agarwal R, Mukhtar H. ENHANCEMENT OF ANTIOXIDANT AND PHASE-II ENZYMES BY ORAL-FEEDING OF GREEN TEA POLYPHENOLS IN DRINKING-WATER TO SKH-1 HAIRLESS MICE - POSSIBLE ROLE IN CANCER CHEMOPREVENTION. Cancer Res. 1992;52:4050–4052. [PubMed] [Google Scholar]
- 94.Mohan K, Hara Y, Abraham SK, Nagini S. Comparative evaluation of the chemopreventive efficacy of green and black tea polyphenols in the hamster buccal pouch carcinogenesis model. Clin Biochem. 2005;38:879–886. doi: 10.1016/j.clinbiochem.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 95.Yu R, Jiao JJ, Duh JL, Gudehithlu K, Tan TH, Kong ANT. Activation of mitogen-activated protein kinases by green tea polyphenols: Potential signaling pathways in the regulation of antioxidant-responsive element-mediated Phase II enzyme gene expression. Carcinogenesis. 1997;18:451–456. doi: 10.1093/carcin/18.2.451. [DOI] [PubMed] [Google Scholar]
- 96.Yuan JH, Li YQ, Yang XY. Inhibition of epigallocatechin gallate on orthotopic colon cancer by upregulating the Nrf2-UGT1A signal pathway in nude mice. Pharmacology. 2007;80:269–278. doi: 10.1159/000106447. [DOI] [PubMed] [Google Scholar]
- 97.Shen GX, Xu CJ, Hu R, Jain MR, Nair S, Lin W, Yang CS, Chan JY, Kong ANT. Comparison of (–)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (–/–) mice. Pharm Res. 2005;22:1805–1820. doi: 10.1007/s11095-005-7546-8. [DOI] [PubMed] [Google Scholar]
- 98.Chow HHS, Hakim IA, Vining DR, Crowell JA, Tome ME, Ranger-Moore J, Cordova CA, Mikhael DM, Briehl MM, Alberts DS. Modulation of human glutathione S-transferases by Polyphenon E intervention. Cancer Epidemiol Biomarkers Prev. 2007;16:1662–1666. doi: 10.1158/1055-9965.EPI-06-0830. [DOI] [PubMed] [Google Scholar]
- 99.Chandronitha C, Ananthi S, Ramakrishnan G, Lakshmisundaram R, Gayathri V, Vasanthi HR. Protective role of tannin-rich fraction of Camellia sinensis in tissue arsenic burden in Sprague Dawley rats. Hum Exp Toxicol. 2010;29:705–719. doi: 10.1177/0960327110361503. [DOI] [PubMed] [Google Scholar]
- 100.Tang LL, Tang M, Xu L, Luo HT, Huang TR, Yu JH, Zhang LS, Gao WM, Cox SB, Wang JS. Modulation of aflatoxin biomarkers in human blood and urine by green tea polyphenols intervention. Carcinogenesis. 2008;29:411–417. doi: 10.1093/carcin/bgn008. [DOI] [PubMed] [Google Scholar]
- 101.Lee D, Khaja S, Velasquez-Castano JC, Dasari M, Sun C, Petros J, Taylor WR, Murthy N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat Mater. 2007;6:765–769. doi: 10.1038/nmat1983. [DOI] [PubMed] [Google Scholar]


