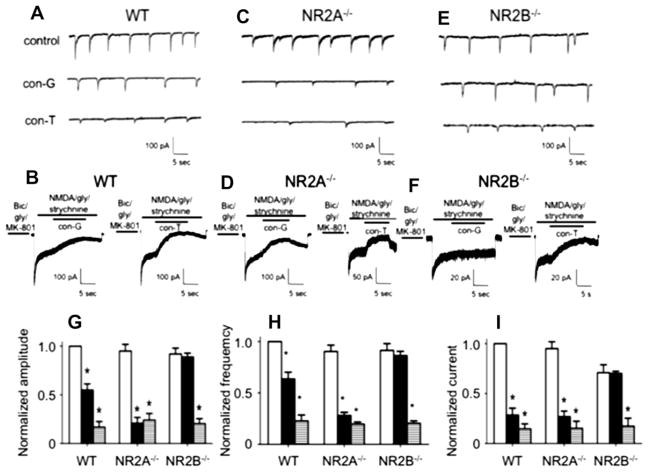
Fig. 7.
Inhibition of synaptic and extrasynaptic currents by con-G and con-T. Comparison of con-G and con-T on sEPSCs (A) and extrasynaptic currents (B) in DIV 13–15 WT mouse cortical neurons. For sEPSCs, bicuculline and CNQX were added to eliminate the fast (early) sEPSCs from AMPA/kainate channels, thus leading to the slow sEPSCs that originate from NMDARs. Inhibition of sEPSCs was achieved by 3 μM con-G or 3 μM con-T. For recording extrasynaptic currents, synaptic NMDARs were blocked with an extracellular bath solution containing bicuculline/gly/MK-801 for 1 min. The current was inhibited with 40 μM con-G or 40 μM con-T. C, D. Comparison of inhibition of sEPSCs (C) and extrasynaptic currents (D) by 3 μM con-G or 3 mM con-T in DIV 13–15 mouse NR2A−/− cortical neurons. E, F. Electrophysiological traces of inhibition by 40 μM con-G or 40 μM con-T of sEPSCs (E) and extrasynaptic currents (F) in DIV 13–15 mouse NR2B−/− cortical neurons. G. Inhibition of peak amplitudes of sEPSC in the absence (control-white bars) or presence of con-G (black bars) or con-T (horizontal striped bars) in WT, NR2A−/−, and NR2B−/− neurons. Data are plotted as the amplitudes normalized to controls (set at 1.0) for each group of neurons, using 6–8 neurons from 3 to 5 independent experiments. H. Inhibition of frequency of sEPSC in the absence (control-white bars) or presence of con-G (black bars) or con-T (horizontal striped bars) in WT, NR2A−/−, and NR2B−/− neurons. Data are plotted as the amplitudes normalized to controls (set at 1.0) for each group of neurons, using 6–8 neurons from 3 to 5 independent experiments. I. Inhibition of extrasynaptic currents in the absence (control-white bars) or presence of con-G (black bars) or con-T (horizontal striped bars) in WT, NR2A−/−, and NR2B−/− neurons. Data are plotted as the currents normalized to controls (set at 1.0) for each group of neurons, using 6–8 neurons from 3 to 5 independent experiments. *p < 0.05, compared with the control (C) within each group.