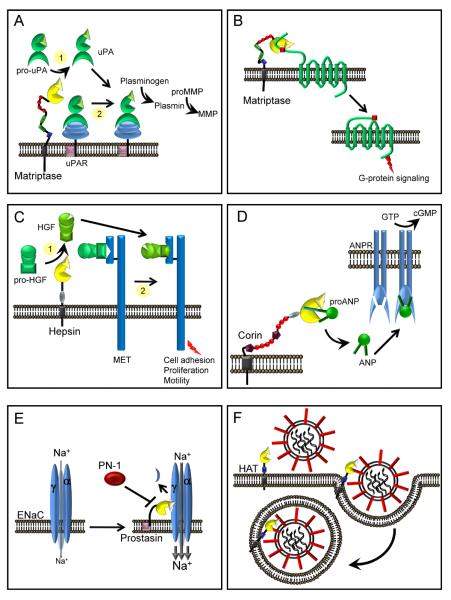
Figure 3. Proteolytic pathways associated with membrane anchored serine proteases in pericellular microenvironments.
(A) Fibrinolysis. The membrane anchored serine proteases, matriptase, hepsin and Serase-1B may participate in the initiation of the plasminogen activation cascade via conversion of pro-uPA to active uPA. Matriptase is a relatively poor activator of pro-uPA in solution (1), whereas it is an effective activator of uPAR-bound pro-uPA and initiates pericellular plasminogen activation on the monocyte cell surface (2). (B) Inflammation. Matriptase and possibly other membrane serine proteases, may participate in the activation of PARs triggering G-protein signaling pathways. (C) Growth factor activation. Hepsin and matriptase may participate in the activation of pro-HGF to initiate signaling via the HGF receptor, MET, to modulate cell adhesion, proliferation and cell motility. Whether these enzymes target soluble pro-HGF (1) or receptor bound pro-HGF (2) is not known. (D) Natriuretic peptides. Corin processes pro-ANP to an active form that bind ANP receptors (ANPR) in the kidney and vasculature, increasing guanylate cyclase activity to regulate blood pressure and blood volume. (E) Sodium homeostasis. The open probability of the ENaC is increased by prostasin and TMPRSS4, resulting in increased cellular uptake of sodium (Na+) which in turn can regulate homeostasis of extracellular fluid volume, blood pressure and Na+ reabsorption. Processing of the α or γ subunits of ENaC is predicted to release an inhibitory fragment that leads to full activation of the channel. The serpin protease nexin-1 (PN-1, serpinE2) can inhibit prostasin mediated activation of ENaC. (F) Viral Pathogenicity. Several TTSPs, including HAT, TMPRSS2, 4, and 13 can process precursor viral proteins that facilitate virus entry and fusion into host cells.