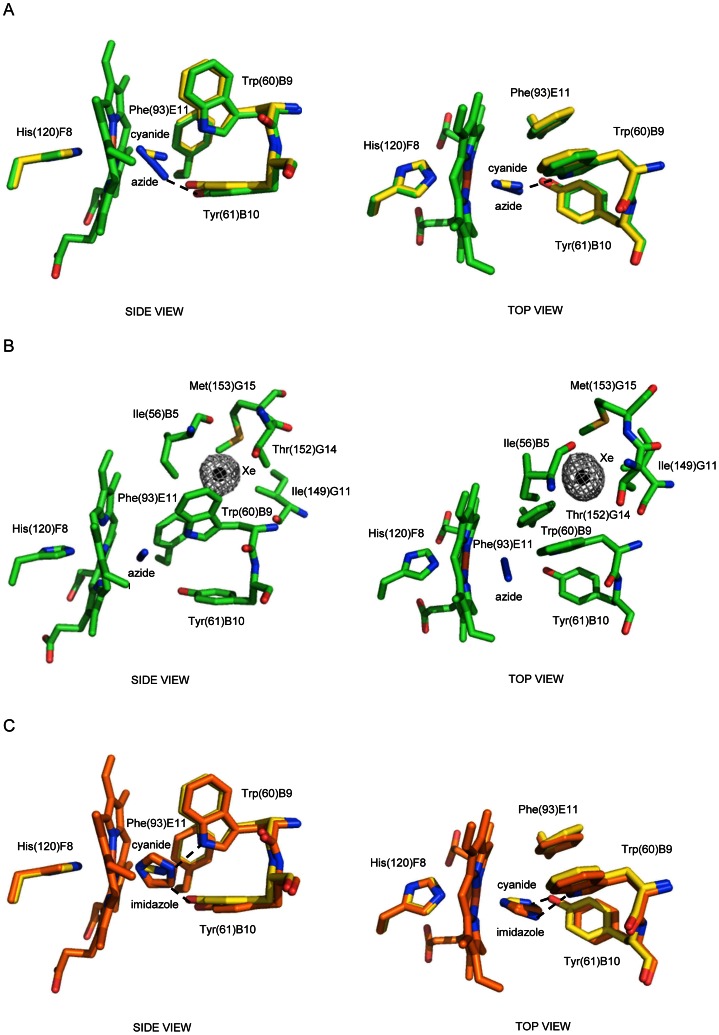
Figure 5. The haem distal site of MaPgb*(III) in complex with azide, azide and Xenon, and imidazole.
Residues lining the haem distal pocket are indicated and shown in stick representation (green) for the MaPgb*(III)-azide structure, and (orange) for the MaPgb*(III)-imidazole structure. (A) Superimposition of MaPgb*(III)-cyanide (yellow) onto the MaPgb*(III)-azide structure. (B) Xenon binding inside tunne1. The Xe atom is shown as a black sphere with the corresponding electron density (2Fo-Fc map contoured at 1σ) shown as grey mesh. (C) Superimposition of MaPgb*(III)-cyanide (yellow) onto the MaPgb*(III)-imidazole structure (the imidazole molecule is shown in two alternate binding modes). In all panels, the proximal His(120)F8 residue is also shown, with the H-bonds to the haem-Fe(III)-bound ligands indicated by dashed lines. All panels are shown from side and top views.