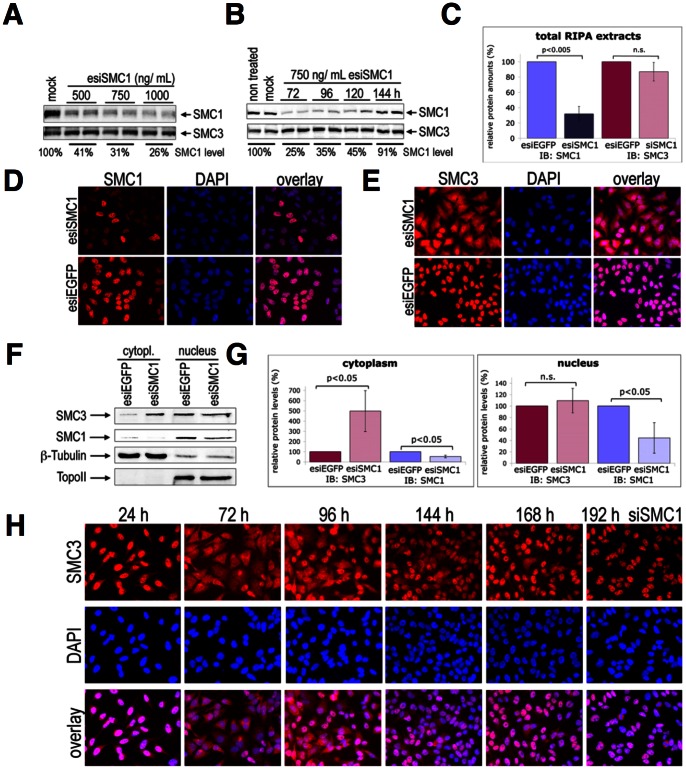
Figure 1. Transient down-regulation of endogenous human SMC1 in HeLa cells using specific esiRNA or siRNA impairs the nuclear localization of SMC3.
(A) RIPA total cell extracts were prepared 72 h after treatment with three different concentrations of esiSMC1 and examined by IB using anti-SMC1 antibody. Mock transfected cells were used as negative control. The membrane was reprobed with anti-SMC3 antibody to confirm the specificity of esiSMC1 and equal loading. The percentages of SMC1 protein levels, normalized to SMC3 protein levels with respect to mock control set at 100%, are indicated. (B) Kinetics of recovery of SMC1 expression after treatment of cells with 750 ng/mL of esiSMC1 was analyzed by IB as described in A. (C) Quantification of SMC1 and SMC3 in RIPA total extracts of cells treated with 750 ng/mL of esiRNA and collected 72 h post transfection. Average of six independent experiments is shown. (D) IF microscopic analysis of SMC1 knockdown 72 h post esiRNA transfection by anti-SMC1 staining in red and DAPI in blue. Specific esiRNA against EGFP (esiEGFP) was used as a control. (E) IF microscopic analyses of esiSMC1- or esiEGFP-treated cells (72 h) using anti-SMC3 (red) and DAPI (blue). (F) Cytoplasmic and nuclear extracts from esiSMC1 or control treated cells were analyzed by IB using anti-SMC3. The membrane was reprobed with anti-SMC1. Anti-ß tubulin and Topo II antibodies were used to determine the purity of nuclear and cytoplasm extracts. (G) Quantification of results from four independent experiments that were performed as described in F. (H) Time course of SMC3 localization upon treatment of cells with siSMC1 (#1) as visualized by IF microscopy using anti-SMC3 (in red) and DAPI (in blue).