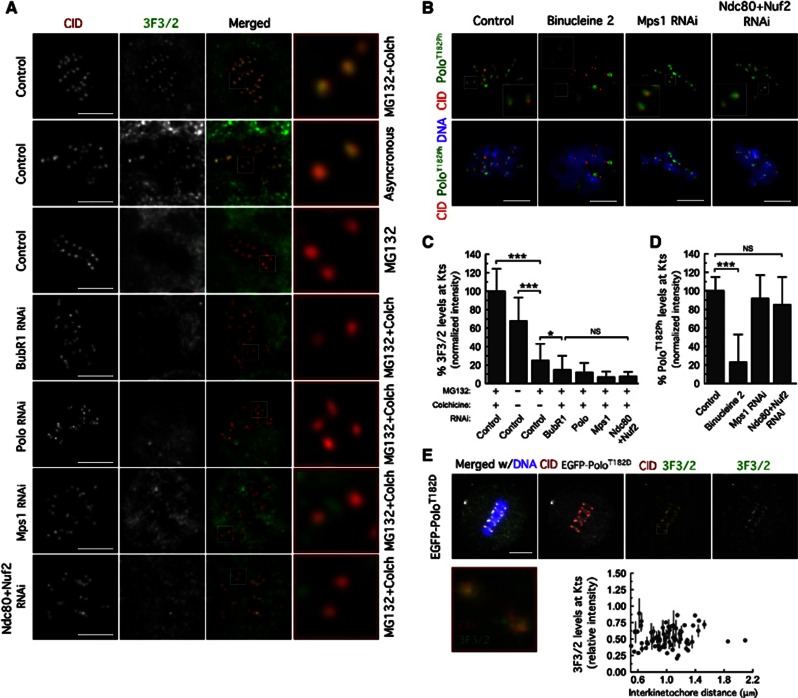
Figure 5.
Mps1 kinetochore localization is required to promote 3F3/2 phosphoepitope formation. (A) Immunolocalization of 3F3/2 phosphoepitope. Kinetochore pairs in boxed areas are shown at higher magnification. (B) Immunofluorescence analysis of Polo-T182 phosphorylation. The insets show higher-magnification views of kinetochore pairs in the boxed areas. Cultured cells were treated with MG132 for 1 h followed by 2 h of colchicine incubation. Binucleine 2 was added to cultures 30 min before microtubule depolymerization with colchicine. (C) Quantification of experiment in (A). 3F3/2 signal intensities at kinetochores were determined relative to CID. Data represent mean±s.d. and the mean values obtained for control cells were set to 100%. 0.01<*P<0.05; ***P<0.001, One-way ANOVA, Turkey’s multiple comparison test. n>20 cells for each condition. (D) Quantifications of experiment in (B). PoloT182Ph fluorescence intensities at kinetochores were determined relative to CID. Data represent mean±s.d. and mean values obtained for control cells were set to 100%. ***P<0.001, One-way ANOVA, Turkey’s multiple comparison test. n>20 cells for each condition. (E) Immunolocalization of 3F3/2 phosphoepitope in S2 cells expressing EGFP–PoloT182D treated with MG132 for 2 h. Kinetochore pair in boxed area is shown at higher magnification. 3F3/2 kinetochore levels were determined relative to CID and plotted against interkinetochore distance. Scale bars represent 5 μm.