Fig. 7.
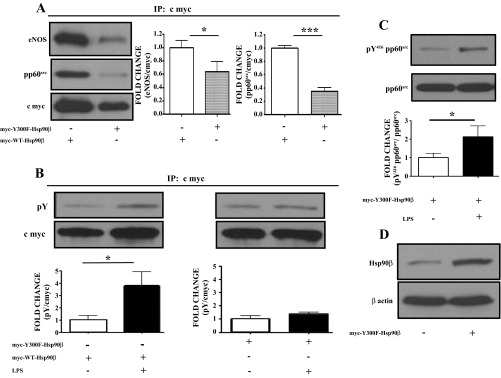
Y300F mutated Hsp90β exhibits reduced ability to bind the Hsp90 client proteins eNOS and pp60src and resists LPS-induced Y phosphorylation in HLMVEC. A: HLMVEC transfected with myc-Y300F-Hsp90β plasmid show a reduced ability to bind the Hsp90 client proteins eNOS and pp60src. Myc-WT-Hsp90β transfected cells were used as control. Band density of eNOS and pp60src was analyzed by densitometry and normalized to the c-myc tag. *P < 0.05, ***P < 0.001. Blots shown represent 1 of 3 independent experiments for eNOS or 4 independent experiments for pp60src. Bars represent SE. B: Western blot analysis of the expression levels of pY in HLMVEC transfected with myc-Y300F-Hsp90β or myc-WT-Hsp90β plasmids and exposed to LPS or vehicle. Band density of pY and c-myc was analyzed by densitometry and normalized to the c-myc tag. *P < 0.05. The blot shown represents 1 of 3 independent experiments. Bars represent SE. C: Western blot analysis of the expression levels of pY416pp60src in HLMVEC transfected with myc-Y300F-Hsp90β and exposed to LPS. Band density of pY416 pp60src was analyzed by densitometry and normalized to pp60src. The blot shown represents 1 of 6 independent experiments. Bars represent SE. D: Western blot analysis of the expression levels of Hsp90β in HLMVEC transfected with myc-Y300F-Hsp90β. The β actin band was used as a loading control.
