Abstract
Hox genes encode transcription factors governing complex developmental processes in several organs. A subset of Hox genes are expressed in the developing lung. Except for Hoxa5, the lack of overt lung phenotype in single mutants suggests that Hox genes may not play a predominant role in lung ontogeny or that functional redundancy may mask anomalies. In the Hox5 paralog group, both Hoxa5 and Hoxb5 genes are expressed in the lung mesenchyme whereas Hoxa5 is also expressed in the tracheal mesenchyme. Herein, we generated Hoxa5;Hoxb5 compound mutant mice to evaluate the relative contribution of each gene to lung development. Hoxa5;Hoxb5 mutants carrying the four mutated alleles displayed an aggravated lung phenotype, resulting in the death of the mutant pups at birth. Characterization of the phenotype highlighted the role of Hoxb5 in lung formation, the latter being involved in branching morphogenesis, goblet cell specification, and postnatal air space structure, revealing partial functional redundancy with Hoxa5. However, the Hoxb5 lung phenotypes were less severe than those seen in Hoxa5 mutants, likely because of Hoxa5 compensation. New specific roles for Hoxa5 were also unveiled, demonstrating the extensive contribution of Hoxa5 to the developing respiratory system. The exclusive expression of Hoxa5 in the trachea and the phrenic motor column likely underlies the Hoxa5-specific trachea and diaphragm phenotypes. Altogether, our observations establish that the Hoxa5 and Hoxb5 paralog genes shared some functions during lung morphogenesis, Hoxa5 playing a predominant role.
Keywords: paralog Hox genes, lung development, Hoxa5, Hoxb5, Hoxc5, functional redundancy
to permit respiratory gas exchanges between blood and environmental air, the lung must have an efficient diffusible interface that results from finely regulated developmental processes. In the mouse, lung ontogeny initiates around embryonic day (E) 9 with the outpocketing of the ventral foregut endoderm into the splanchnic mesenchyme to form the laryngotracheal groove. The lung bud then elongates and undergoes a stereotypic pattern of branching leading to the formation of the respiratory tree. Starting at E16.5, the prospective gas-exchange region develops and capillaries get apposed to the flattened respiratory epithelium to form the first blood-air barriers. Lung development culminates postnatally from day (D) 5 to D30 with the formation of alveoli, the final gas-exchange units. Throughout lung formation, undifferentiated epithelial cells present in the primary buds proliferate and differentiate into specialized cell types regionally located along the proximodistal axis of the respiratory tract (38, 55).
Lung morphogenesis is influenced by physical forces. For instance, impairment of fetal breathing movements due to a nonfunctional diaphragm can cause lung hypoplasia (25, 27, 32). Moreover, lung development is under the concerted action of multiple molecules organized in distinct networks that control the patterning of the respiratory tract (38). Reciprocal interactions between lung mesenchyme and its flanking epithelium also govern lung morphogenesis and epithelial cell fate determination (48). Despite accumulated evidence showing that mesenchymal cells can instruct the epithelium, the nature of the mesenchymal factors involved has yet to be resolved.
Hox genes encode transcription factors specifying the regionalization of the body plan and regulating morphogenesis during animal development. In human and mouse, 39 Hox genes are organized in four clusters located on different chromosomes. The 3′ to 5′ position of each Hox gene within a cluster corresponds to its spatiotemporal expression domain along the anterior-posterior axis of the embryo. Different members of the Hox complexes are thus expressed in overlapping domains along the developing body, suggesting that specific combinations of HOX proteins provide a unique address to a particular region. On the basis of sequence homologies and position within clusters, Hox genes are also classified into 13 paralog groups (34). The similarities in protein structure and expression pattern among genes from the same paralog group have led to the hypothesis that Hox paralogs perform partially redundant and/or overlapping functions. Indeed, compound mutant mice for Hox paralogs often exhibit a more severe phenotype than mutant mice for a single Hox gene (10, 35, 44, 45, 56). Furthermore, knock-in substitutions of Hox genes by their paralogs have demonstrated that they can fulfill similar roles (22, 51).
In the lung, the proximodistal distribution of the different structures (trachea, bronchi, bronchioli, alveoli) and cell types suggests that the respiratory tract can be specified by Hox-regulated mechanisms similar to those governing anterior-posterior axial skeleton formation. Hox genes, predominantly from paralogous groups 1 to 8, are mainly expressed in lung mesenchyme with a distinct spatiotemporal profile, supporting a role in the regional specification of the respiratory tract (5, 26). However, except for Hoxa5, the lack of overt lung phenotype in single mutants suggests that Hox genes may not play a predominant role in lung ontogeny or that functional redundancy may mask anomalies.
Hoxa5, Hoxb5, and Hoxc5 are members of the Hox5 paralog group. Hoxb5 and Hoxc5 mutant mice are viable and no organ defect has been described (8, 43). In contrast, the loss of Hoxa5 function results in a panoply of phenotypes indicative of the broad range of Hoxa5 actions throughout life, including tracheal and lung dysmorphogenesis responsible for the high neonatal mortality rate of Hoxa5−/− pups (1, 2, 4, 17, 19, 29, 36). Surviving Hoxa5−/− mice display an emphysema-like phenotype characterized by air space enlargement and goblet cell metaplasia (33).
Even though no lung phenotype was reported in Hoxb5 mutant mice, Hoxb5 lung expression, in vitro studies, and expression data in human lung diseases suggest a role for Hoxb5 in lung development (6, 43, 52, 53). The severity of the Hoxa5 lung phenotype indicates that Hoxa5 function is less subject to rescue by other genes. However, it is possible that the other Hox5 paralogs exert functions in lung development hidden by Hoxa5 compensation. A threshold level of HOX5 proteins may also be required for specific aspects of development, and mutation of several paralogs may be needed to reveal defects otherwise not detectable (10, 35, 45). Herein, genetic interactions between Hoxa5 and Hoxb5 paralog genes during lung morphogenesis were investigated. Our in vivo data confirm the importance of Hoxa5 and uncovers the role of Hoxb5 in lung morphogenesis. Hoxa5 and Hoxb5 genes share functions in the developing lung, Hoxa5 playing a predominant role.
MATERIALS AND METHODS
Mice, genotyping, and tissue collection.
The Hoxa5 and Hoxb5 mutant mouse lines were maintained in the 129/Sv inbred background. Hoxa5−/− mice were mated with Hoxb5−/− mice to obtain double heterozygous animals (Hoxa5+/−;Hoxb5+/−). The latter were intercrossed to generate mice of all possible allelic combinations. Age of the embryos was estimated by considering the morning of the day of the vaginal plug as E0.5. Experimental animals were genotyped by Southern blot analysis (29, 43).
Lungs were collected from wild-type (WT) and mutant embryos at E13.5, E15.5, and E18.5 and from adults at D65 ± 5. The wet lung and body weights were obtained by direct measurement to determine the ratio. Excised lungs were fixed in 4% cold paraformaldehyde, paraffin embedded, and sectioned at 4 μm (28). For adult specimens, lungs were instilled with 4% cold paraformaldehyde before processing. Diaphragms were dissected from E18.5 embryos and fixed in 1% paraformaldehyde before processing for immunofluorescence (IF) staining. For RNA extraction, lungs were snap frozen in N2. Animal experimentations were performed according to the guidelines of the Canadian Council on Animal Care and approved by the institutional animal care committee.
Histology, IHC, and IF analyses.
Lung sections were stained according to standard histological procedures: hematoxylin and eosin for morphology, Alcian blue for detection of mucus-producing goblet cells, and Weigert with tartrazine counterstaining to visualize elastic fibers. Immunohistochemistry (IHC) experiments were performed as described (19). Slides were counterstained with either Alcian blue and nuclear fast red or methyl green. For phospho-histone H3 (pHH3) immunostaining, ratio of positive-stained cells to total cell number was evaluated for a minimum of six random areas with a total of at least 2,200 cells per specimen. Whole mounts of diaphragms were IF stained to label axons and nerve terminals (24). The primary antibodies used were a rabbit monoclonal antibody against pHH3 (1/200 dilution; Cell Signaling), a rabbit antibody against cleaved caspase-3 (1/200; Cell Signaling), a goat antibody against CC10 (1/500; gift from Dr. G. Singh), a Syrian hamster monoclonal antibody against podoplanin (T1α; 1/1,000; DSHB), a goat antibody against FOXA2 (1/100; Santa Cruz Biotechnology), a rabbit antibody against pro-SP-C (1/500; Millipore), a rat monoclonal against PECAM-1 (1/75; BD Biosciences Pharmingen), a rabbit antibody against neurofilament (1/1,000; Millipore), a rabbit antibody against synaptophysin (1/5; Invitrogen), a rabbit antibody against N1ICD (1/250; Abcam), and a rabbit antibody against HEY2 (1/250; Millipore). The biotinylated secondary antibodies used were a goat anti-rabbit antibody (1/300; Vector Laboratories), a swine anti-goat antibody (1/300; Cedarlane), a goat anti-Syrian hamster antibody (1/300; Cedarlane), and a goat anti-rat antibody (1/500; Cedarlane). The fluorescent secondary antibodies FITC-conjugated donkey anti-goat IgG (1/250; Cedarlane) and Alexa Fluor 488-conjugated donkey anti-rabbit IgG (1/1,000; Invitrogen) were also used. Finally, the Alexa Fluor 555-conjugated α-bungorotoxin (1/1,000; Invitrogen) was used.
Morphometry.
Radial alveolar count, a measure of the complexity of the respiratory acini, was determined by counting the number of air spaces lying on a line drawn perpendicularly from the center of a terminal or respiratory bronchiole to the closest edge of the pleural tissue (15). For tracheal luminal measurement, images of histological sections of WT and mutant specimens were visualized with a Leica SCN400 slide scanner (Leica Microsystems, Concord, ON, Canada). The apical epithelial surface of each image was traced and quantified by using the Adobe Photoshop CS3 software.
In situ hybridization analyses.
Radioactive in situ hybridization experiments were performed on frontal sections of E13.5 embryos as described (28). The following murine sequences were used as specific templates for synthesizing [35S]UTP-labeled riboprobes: a 850-bp BglII-HindIII genomic fragment containing the 3′-untranslated region of Hoxa5 exon 2 (11), a 430-bp BamHI-HindIII cDNA fragment covering part of Hoxb5 exon 1 (31), and a 200-bp EcoRI-XbaI genomic fragment corresponding to Hoxc5 exon 2 (18). After exposure, slides were counterstained with toluidine blue. Experiments were performed on two to five specimens per genotype tested.
qRT-PCR experiments.
Total RNA was isolated from the trachea/primary bronchi and the lungs of E18.5 embryos and from the entire respiratory tract of E15.5 embryos according to the TRIzol reagent procedure (Invitrogen, Carlsbad, CA). cDNA was synthesized with the Superscript II Reverse Transcriptase (Invitrogen) using random primers. Quantitative (q)PCR was performed with Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) and a thermal cycler ABI PRISM 7000. Samples were analyzed in triplicate. Five to eight specimens were used for each genotype tested. Primer sequences used were as follows: Fstl1 forward ATGGCGACTCTCACCTGGAC, reverse CAATGAGGGCGTCAACACAG; Hoxa5 forward CCCAGATCTACCCCTGGATG, reverse GGCATGAGCTATTTCGATCCT; Hoxb5 forward TATTCCCCTGGATGAGGAAG, reverse GGGTCAGGTAGCGATTGAAG; T1α forward TGGCAAGGCACCTCTGGTA, reverse GGTGGACAGTTCCTCTAAGGGA; Vegfa forward TGCACTGGACCCTGGCTTTAC, reverse CGGCAGTAGCTTCGCTGGTAG. The Rpl19 gene was used as control with the primers forward GATCATCCGCAAGCCTGTGA and reverse GCATCCGAGCATTGGCAGTA.
ChIP assays.
Trachea from E18.5 WT embryos was isolated and mechanically disrupted prior to be cross-linked with 1% formaldehyde in PBS for 15 min at room temperature. Cross-linking was stopped by adding glycine to a final concentration of 0.125 M. Extracts were then disrupted into crude lysates in 2 ml of swelling buffer (5 mM PIPES pH 8.0, 85 mM KCl, 1% Nonidet P-40, and protease inhibitors), equilibrated 20 min on ice, and centrifuged for 5 min at 3,000 rpm at 4°C. The pellets were eluted in 1 ml of chromatin immunoprecipitation (ChIP) lysis buffer (50 mM Tris·HCl pH 8.0, 10 mM EDTA, 1% SDS) with protease inhibitors (Complete Mini-EDTA-free; Roche Diagnostics, Mannheim, Germany). Sonication was performed by using a Bioruptor (Diagenode, Liège, Belgium) for 10 cycles of a 30-s pulse followed by a 30-s pause at the highest setting to obtain an average DNA size of 300–600 bp. Fragmented chromatin (100 μg) was incubated overnight at 4°C with Dynabeads linked to protein G (Invitrogen) and 2 μg of either rabbit anti-HOXA5 antibody (Aviva Systems Biology, San Diego, CA), rabbit anti-histone H3 (ab1791; Abcam, Cambridge, MA), or rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). Postimmunoprecipitation washes, chromatin elution, cross-link reversion, and purification of the DNA fragments were done prior to qPCR amplification as described (41). qPCR-ChIP analyses were performed with the following primers: Fstl1-1 forward AATACTGCCACTGGCCACTAACCA, reverse CTTTGGTCCCATGCCTTTGGGTTT; Fstl1-2 forward AACACAGGTCCACCAGCTTGTATG, reverse GACATGTGATAGTCAATACGGTGC; Fstl1-3 forward ACAGACTGTAGCTCTCAGGAAGCA, reverse TGGGACAAGGGCTTGATTTAGGGA; Fstl1-4 forward ACTCTGAATCAAGGAGAATTATGTCCTA, reverse CCAGAGCTTGCAACATGCCGTATT; ctl locus forward TGGAGTGCTTACAAGGAAGGCAGA, reverse ACTAACCGCCATGTTCTTTCGCTC. The values for the samples immunoprecipitated by anti-HOXA5, anti-histone H3, or control IgG were recorded as the percentage relative to input. ChIP results were confirmed by three independent experiments and qPCR was performed in triplicate for each sample.
Statistical analyses.
Student's t-test was performed to compare the lung weight-to-body weight ratio, the number of pHH3-positive cells, the number of acini, the CC10 intensity staining, the number of tracheal rings, the luminal surface of the trachea, the radial alveolar counts as well as for gene expression experiments. A significance level inferior to 5% (P < 0.05) was considered statistically significant.
RESULTS
Expression of Hox5 paralog genes in the developing lung.
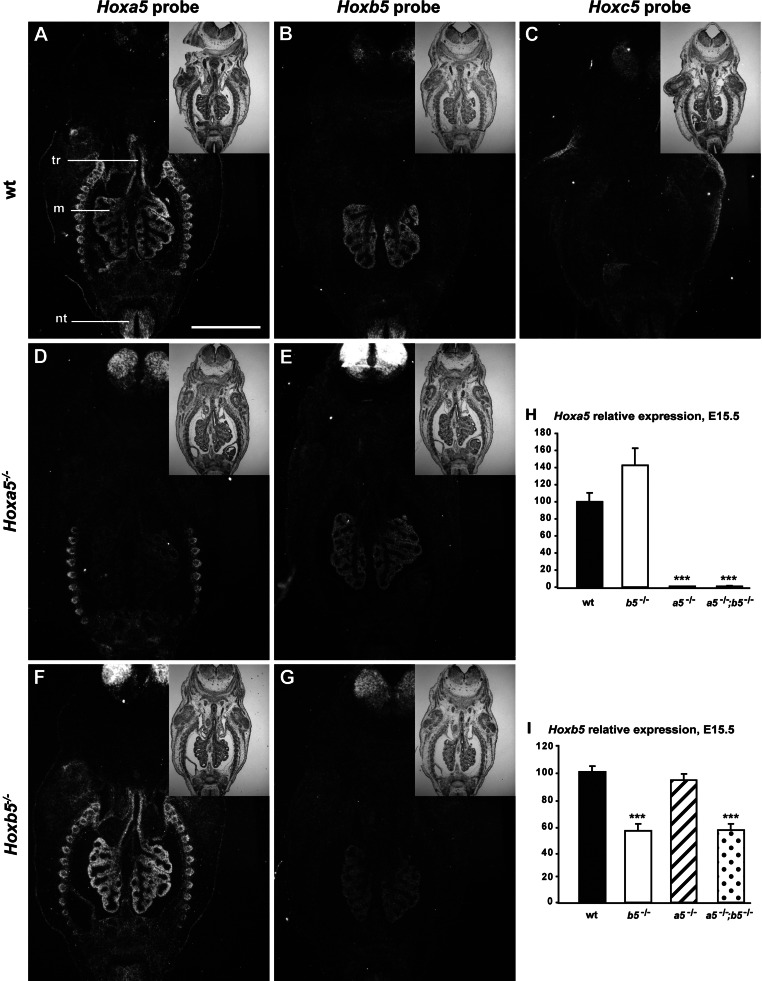
To define whether the three Hox5 paralog genes share a similar expression profile along the respiratory tract, we performed comparative in situ hybridization experiments on lungs from E13.5 embryos using specific riboprobe for each gene. Hoxa5 and Hoxb5 transcripts were readily seen throughout the mesenchymal component of the distal lung, whereas only Hoxa5 expression was observed in the mesenchyme surrounding the epithelium of the trachea and the primary bronchi (Fig. 1, A and B). No evident Hoxc5 expression was detected along the respiratory tract (Fig. 1C). Thus only Hoxa5 and Hoxb5 genes displayed specific and overlapping expression domains in the developing lung. Therefore, we focused our attention on these two Hox5 paralog genes.
Fig. 1.
Expression analyses of Hox5 paralog genes in the developing lung. In situ hybridization was performed on frontal sections of embryonic day (E)13.5 wild-type (WT) (A–C), Hoxa5−/− (D, E), and Hoxb5−/− (F, G) mouse embryos with Hoxa5 (A, D, F), Hoxb5 (B, E, G), and Hoxc5 (C) specific riboprobes. Representative bright-field (insets) and dark-field images are shown. A strong Hoxa5 signal was detected in the mesenchyme surrounding the entire respiratory tract from the trachea to the distal lung. Hoxb5 transcripts were restricted to the distal lung mesenchyme, whereas no Hoxc5 expression was observed in the lung. m, Mesenchyme; nt, neural tube; tr, trachea. Scale bar: 500 μm. H and I: quantitative (q)RT-PCR analysis of Hoxa5 (H) and Hoxb5 (I) expression levels in the entire respiratory tract (from trachea to lungs) of E15.5 embryos. Hoxa5 expression was upregulated in Hoxb5−/− specimens suggesting Hoxa5 compensatory effects. No change in Hoxb5 expression was observed in Hoxa5 mutants. The decreased Hoxa5 expression in lungs from Hoxa5−/− embryos and the reduced Hoxb5 expression in Hoxb5−/− specimens might reflect the interference of the neo selection cassette on Hox transcription. Values are expressed as means ± SE. ***P < 0.001.
The loss of Hoxb5 alleles reduces the viability of Hoxa5−/− pups.
Given that Hox5 paralog genes demonstrate functional redundancy in axial patterning, we assessed whether Hoxb5 plays a role in lung development that can be masked by Hoxa5 compensation (35). In situ hybridization and qRT-PCR experiments revealed an increased Hoxa5 expression in Hoxb5−/− specimens that supported this notion (Fig. 1, F and H). In contrast, Hoxb5 expression remained unchanged in lungs from Hoxa5−/− embryos (Fig. 1, E and I). Even though mutant transcripts are produced from the Hoxa5 and Hoxb5 mutant alleles, both Hoxa5 and Hoxb5 mutations generate a null allele with no production of protein (11, 43). We observed a loss of Hoxa5 expression in lungs from Hoxa5−/− embryos and a reduced Hoxb5 expression in Hoxb5−/− specimens that might reflect the interference of the neo selection cassette on Hox transcription as reported (Fig. 1, D and G–I; Ref. 3).
We generated Hoxa5;Hoxb5 compound mutant mice. At weaning age, only 2% of Hoxa5−/− mice were recovered instead of the expected 6.25%, reflecting the high rate (66%) of lethality associated with the Hoxa5 mutation (Table 1; Ref. 29). The survival of animals decreased with the number of Hoxb5 mutant alleles: less than 10% of the expected number of Hoxa5−/−;Hoxb5+/− mice and no double mutants Hoxa5−/−;Hoxb5−/− were recovered at weaning. Timed matings showed that, during gestation, specimens of all nine possible genotypes were obtained at the expected Mendelian ratio. However, a close monitoring of the litters at birth revealed a high rate of mortality of the compound mutants deficient for both Hoxa5 alleles and demonstrated that the loss of Hoxb5 function worsened the neonatal lethality phenotype of the Hoxa5 mutation.
Table 1.
Ratios of genotypes of litters from intercrosses of Hoxa5+/−; Hoxb5+/− mutant mice
| Genotypes* |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | No. of litters | No. of animals | Hoxa5+/+; Hoxb5+/+ | Hoxa5+/+; Hoxb5+/− | Hoxa5+/+; Hoxb5+/− | Hoxa5+/−; Hoxb5+/+ | Hoxa5+/−; Hoxb5+/− | Hoxa5+/−; Hoxb5+/− | Hoxa5−/−; Hoxb5+/− | Hoxa5−/−; Hoxb5+/− | Hoxa5−/−; Hoxb5−/− |
| E13.5 | 24 | 138 | 7 | 15 | 11 | 19 | 30 | 27 | 8 | 12 | 9 |
| % obtained | 5% | 11% | 8% | 14% | 22% | 20% | 6% | 9% | 7% | ||
| E15.5 | 20 | 125 | 9 | 20 | 7 | 15 | 35 | 11 | 7 | 16 | 5 |
| % obtained | 7% | 16% | 6% | 12% | 28% | 9% | 6% | 13% | 4% | ||
| E18.5 | 63 | 325 | 18 | 36 | 33 | 45 | 68 | 52 | 16 | 37 | 20 |
| % obtained | 6% | 11% | 10% | 14% | 21% | 16% | 5% | 11% | 6% | ||
| D0 | 41 | 181 (62) | 10 | 25 | 17 | 31 (2) | 65 (4) | 25 | 5 (7) | 3 (21) | 0 (28) |
| % obtained | 4% | 10% | 7% | 12.7% (0.8%) | 26.7% (1.7%) | 10% | 2.1% (2.9%) | 1.2% (8.6%) | 0% (11.5%) | ||
| D21–D28 | 54 | 242 | 14 | 39 | 23 | 37 | 85 | 35 | 6 | 3 | 0 |
| % obtained | 6% | 16% | 10% | 15% | 35% | 14% | 2% | 1% | 0% | ||
| % Expected | 6.25% | 12.50% | 6.25% | 12.50% | 25% | 12.50% | 6.25% | 12.50% | 6.25% | ||
Numbers in parentheses correspond to pups found dead at birth. E, embryonic day; D, day.
An exacerbated lung hypoplasia underlies the increased neonatal lethality of Hoxa5;Hoxb5 compound mutants.
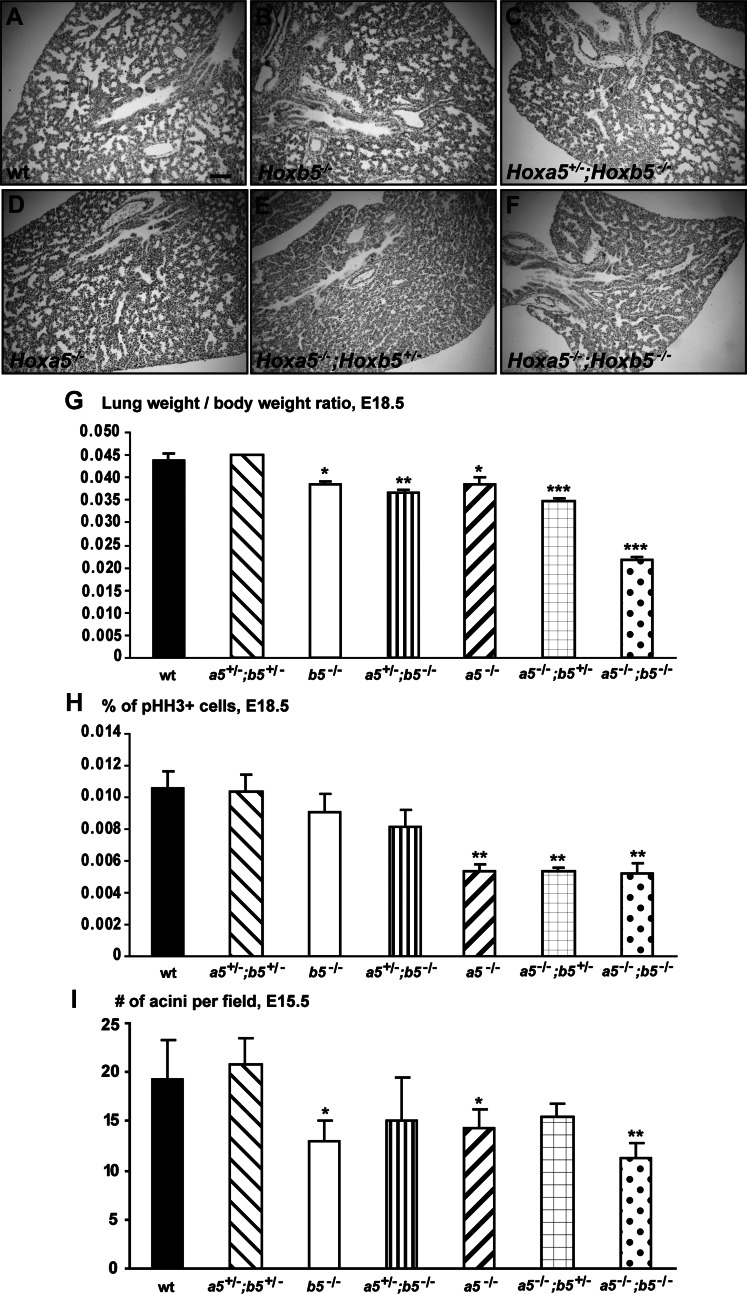
To identify the cause(s) of the higher rate of death of the Hoxa5;Hoxb5 mutants, we verified whether the lung defects associated with the Hoxa5 mutation were aggravated by the reduction of Hoxb5 dosage. At E18.5, the number of lobes and their orientation were preserved in compound mutants indicating that the first bronchial ramifications occurred correctly. Comparative lung morphology of E18.5 embryos revealed that WT, Hoxb5−/−, and Hoxa5+/−;Hoxb5−/− specimens presented a normal structure with dilated peripheral lung saccules and thin mesenchyme (Fig. 2, A–C). However, lungs from Hoxa5−/− embryos exhibited narrower air spaces and thicker mesenchyme as reported (4). The defects appeared more pronounced in Hoxa5−/−;Hoxb5+/− and Hoxa5−/−;Hoxb5−/− embryos (Fig. 2, D–F).
Fig. 2.
Comparative analyses of lungs from Hoxa5;Hoxb5 mutant embryos. A–F: comparative lung histology of E18.5 Hoxa5;Hoxb5 embryos. WT, Hoxb5−/−, and Hoxa5+/−;Hoxb5−/− specimens presented a normal lung structure with dilated peripheral saccules and thin mesenchyme, whereas lungs from Hoxa5−/− embryos exhibited narrower air spaces and thicker mesenchyme. The phenotype worsened with the number of Hoxb5 mutant alleles, and lungs from Hoxa5−/−;Hoxb5−/− embryos were smallest. Scale bar: 100 μm. G: at E18.5, the lung weight-to-body weight ratio was statistically lower for Hoxa5−/−, Hoxb5−/−, Hoxa5+/−;Hoxb5−/−, Hoxa5−/−;Hoxb5+/−, and Hoxa5−/−;Hoxb5−/− embryos compared with WT. H: lung hypoplasia in E18.5 Hoxa5−/−, Hoxa5−/−;Hoxb5+/− and Hoxa5−/−;Hoxb5−/− embryos correlated with a statistically reduced proliferation in the lung. I: lung branching at E15.5, as estimated by the number of acini per field, was statistically less extensive in Hoxa5−/−, Hoxb5−/− and Hoxa5−/−;Hoxb5−/− embryos compared with WT. Values are expressed as means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
At E18.5, the lung weight-to-body weight ratio was significantly diminished in Hoxa5−/−, Hoxb5−/−, Hoxa5+/−;Hoxb5−/−, Hoxa5−/−;Hoxb5+/−, and Hoxa5−/−;Hoxb5−/− embryos (Fig. 2G). Lungs from Hoxa5−/−;Hoxb5−/− embryos were considerably smaller and the lung weight-to-body weight ratio was ∼50% of that of WT embryos. To identify the factors contributing to pulmonary hypoplasia, we measured cell proliferation (Fig. 2H). At E18.5, a statistically reduced number of pHH3-stained lung cells was obtained for the Hoxa5−/−, Hoxa5−/−;Hoxb5+/−, and Hoxa5−/−;Hoxb5−/− genotypes compared with controls, indicating that decreased lung proliferation was specifically associated with the Hoxa5 mutation. Comparable results were obtained at E15.5 (not shown). Immunostaining for the activated form of caspase-3 did not show any significant variations in the extent of apoptosis in E18.5 embryonic lungs between all genotypes (not shown).
As reduced lung branching may participate to lung hypoplasia, the number of acini was evaluated during the pseudoglandular stage at E15.5 (9). At this stage, the ramifying bronchial tree gives rise to tubular branches ending in terminal acini. As previously observed, branching was less extensive in Hoxa5−/− embryos with a mean reduction of 27% (Fig. 2I; Ref. 4). Hoxb5−/− mutants also presented a reduced number of acini indicating that both genes are involved in the branching process. In Hoxa5−/−;Hoxb5−/− specimens, the phenotype was amplified. Thus insufficient proliferation and decreased branching likely participate to the important lung hypoplasia that may explain the increased lethality of Hoxa5−/−;Hoxb5−/− pups at birth.
Hoxa5 and Hoxb5 genes are involved in lung epithelial cell differentiation.
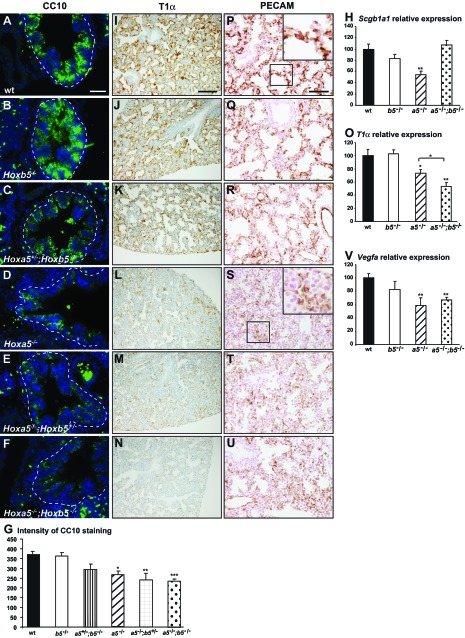
The role of Hoxb5 in lung branching raised the possibility that Hoxb5 can also be involved in other aspects of lung development. To assess the impact of the Hoxa5;Hoxb5 compound mutations on the integrity of the airway epithelium, we examined the expression of specific cell markers in lungs from E18.5 embryos. Decreased expression of the club cell (Clara cell) marker CC10 (encoded by the Scgb1a1 gene) was observed in lung airways of embryos carrying two Hoxa5 mutant alleles, as shown by IF staining and CC10 intensity measurement by using ImageJ as well as by qRT-PCR (Fig. 3, A–H). The decreased CC10 immunostaining in Hoxa5−/−;Hoxb5−/− specimens did not correlate with the Scgb1a1 high expression obtained by qRT-PCR. One likely explanation could be that the important hypoplasia of Hoxa5−/−;Hoxb5−/− lung specimens causes an increase in the proportion of airway epithelial cells vs. the total lung tissue obtained for RNA extraction.
Fig. 3.
Characterization of lung epithelium and microvasculature of E18.5 Hoxa5;Hoxb5 mutant embryos. A–F: as assessed by immunofluorescence (IF), CC10 expression was decreased in lung airways of embryos carrying 2 Hoxa5 mutant alleles indicating the predominant role of Hoxa5 in club cell specification. G: mean CC10 intensity within the airway epithelium was measured by use of ImageJ. Values represent the average fluorescence intensity of the bronchial epithelial areas (surrounded by a dotted line). At least 4 pictures per specimen from 3–5 specimens were measured for each genotype. Values are expressed as means ± SE. I–N: immunostaining with podoplanin (T1α), a marker of type I pneumocytes, was considerably decreased in lungs from Hoxa5−/−, Hoxa5−/−;Hoxb5+/− and Hoxa5−/−;Hoxb5−/− embryos. P–U: PECAM-1 immunostaining revealed an undeveloped vascular network embedded in the thick lung mesenchyme in Hoxa5−/−, Hoxa5−/−;Hoxb5+/−, and Hoxa5−/−;Hoxb5−/− embryos. Scale bars: 100 μm (I–N), 50 μm (A–F, P–U). H, O, V: qRT-PCR analysis for Scgb1a1, T1α, and Vegfa expression in lungs from WT, Hoxa5−/−, Hoxb5−/−, and Hoxa5−/−;Hoxb5−/− E18.5 embryos. Comparison was made against WT. O: T1α expression was also significantly reduced in Hoxa5−/−;Hoxb5−/− specimens compared with Hoxa5−/− samples. Values are expressed as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
We looked at the expression of podoplanin (T1α), a marker of type I pneumocytes, which are flat epithelial cells lining the air spaces and closely interacting with the underlying vascular endothelium for gas exchanges (42). T1α expression was comparable between WT, Hoxb5−/−, and Hoxa5+/−;Hoxb5−/− embryos (Fig. 3, I–K). In contrast, it was dramatically decreased in lungs from Hoxa5−/− embryos, a phenotype accentuated in Hoxa5−/−;Hoxb5−/− mutants as confirmed by qRT-PCR analysis (Fig. 3, L–O). Thus Hoxa5 plays a predominant role in the specification of type I pneumocytes, and Hoxb5 also seems to collaborate.
To investigate whether lung vascular development was also perturbed, we examined endothelial cells using the platelet endothelial cell adhesion molecule 1 (PECAM-1) marker. A growing microvasculature network was observed in the well-developed saccules of WT, Hoxb5−/− and Hoxa5+/−;Hoxb5−/− specimens with staining in close proximity to the lumen (Fig. 3, P–R). In contrast, a faint PECAM-1 staining embedded in a thick mesenchyme was detected in Hoxa5;Hoxb5 compound mutants carrying two Hoxa5 mutant alleles, indicating a relatively undeveloped pulmonary capillary bed (Fig. 3, S–U). The VEGF pathway is involved in driving the epithelial to endothelial cross talk during lung morphogenesis (14). qRT-PCR expression analysis revealed significantly reduced Vegfa expression levels in Hoxa5−/− and Hoxa5−/−;Hoxb5−/− specimens (Fig. 3V). This suggests that Vegfa downregulation might contribute to the abnormal lung microvasculature and supports the notion that this phenotype was mainly associated with the Hoxa5 mutation.
Tracheal malformations are specific to Hoxa5 mutant embryos.
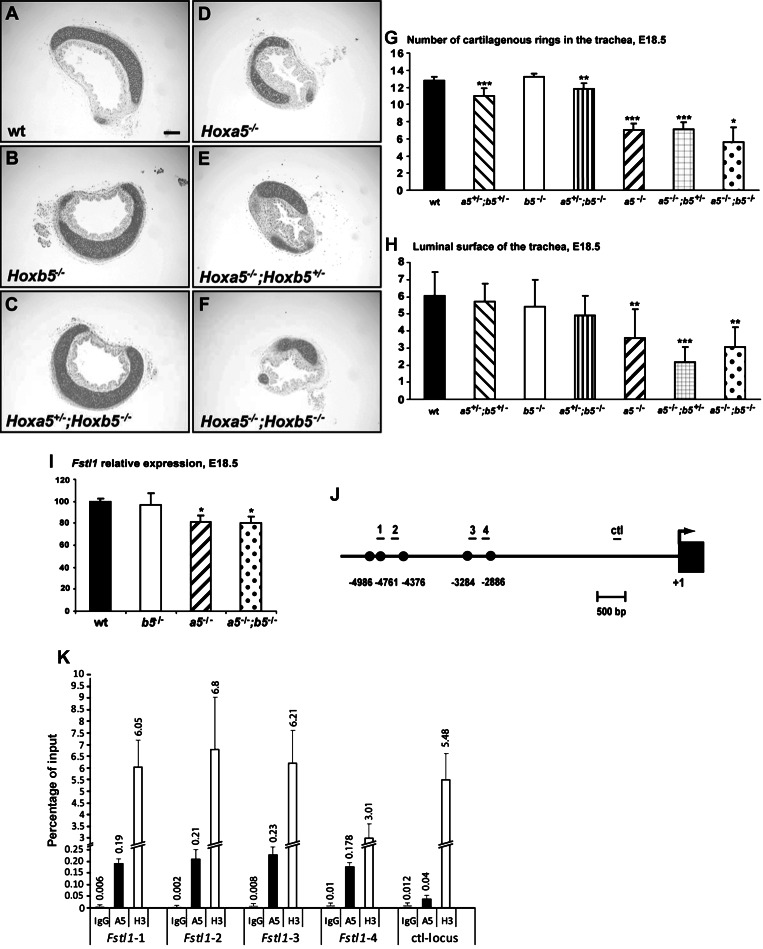
Hoxa5−/− mice exhibit tracheal malformations characterized by disorganized banding pattern and narrowing of the trachea (4). Even though Hoxa5 is the only Hox5 member to be expressed in the upper respiratory airways, a possible worsening of the phenotype was investigated in E18.5 Hoxa5;Hoxb5 embryos. Macroscopic examination of whole trachea and analysis of transverse sections showed a reduced number of cartilaginous rings and a diminished luminal surface in Hoxa5;Hoxb5 mutants carrying two Hoxa5 mutant alleles, independently of the Hoxb5 mutation (Fig. 4, A–H). Thus correct tracheal formation is specifically under the control of the Hoxa5 gene.
Fig. 4.
Hoxa5 plays a predominant role in tracheal formation and it regulates Fstl1 expression in the developing trachea. A–F: transverse sections of trachea from E18.5 Hoxa5;Hoxb5 embryos were Alcian blue stained for morphology analysis. The trachea from Hoxa5−/−, Hoxa5−/−;Hoxb5+/−, and Hoxa5−/−;Hoxb5−/− embryos was narrower and had a diminished luminal surface. Scale bar: 100 μm. G: reduced number of cartilage rings was observed in Hoxa5−/− specimens independently of the Hoxb5 mutation. H: the surface of the lumen was quantified and the numbers correspond to arbitrary units. Values are expressed as means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. I: qRT-PCR analysis of Fstl1 expression revealed diminished levels in trachea/primary bronchi from Hoxa5−/− and Hoxa5−/−;Hoxb5−/− embryos compared with WT. Values are expressed as means ± SE. *P < 0.05. J: schematic representation and position relative to the transcription start site of the 5 putative HOX binding sites in the Fstl1 promoter. The position of the qPCR fragments is indicated. K: ChIP analysis of endogenous Fstl1 regulatory sequences in E18.5 trachea. Chromatin was immunoprecipitated with rabbit IgG, anti-HOXA5 and anti-histone H3 antibodies. Recruitment of HOXA5 and H3 on regulatory sequences of the Fslt1 locus was evaluated by qPCR and is indicated as the percentage of input. HOXA5 binds to the Fstl1 genomic region spanning the HOX binding sites whereas no binding was observed with the Fstl1 DNA negative control. The data are means ± SD of 3 independent experiments.
Hoxa5 controls Fstl1 expression during trachea formation.
Mutation in the follistatin-like 1 (Fstl1) gene, which encodes a bone morphogenetic protein (BMP) antagonist, causes a malformed trachea with a reduced number of rings and a disorganized epithelial layer reminiscent of the Hoxa5 mutant phenotype (20). We monitored Fstl1 expression in the upper airways (trachea and primary bronchi) of E18.5 Hoxa5;Hoxb5 embryos by qRT-PCR. Consistent with our morphological observations, decreased Fstl1 expression was observed in Hoxa5−/− and Hoxa5−/−;Hoxb5−/− specimens but not in Hoxb5−/− ones (Fig. 4I).
Fstl1 is expressed in the mesenchyme surrounding the airways like Hoxa5 (49). Sequence analysis of the mouse Fstl1 gene with the Transcription Element Search System (TESS) software revealed five HOX binding sites into a 2.1-kb region located between positions −5.0 and −2.9 kb upstream the Fstl1 transcription start site (Fig. 4J). To determine whether Fstl1 could be a potential HOXA5 transcriptional target, we performed ChIP assays on cross-linked chromatin isolated from trachea of E18.5 WT mouse embryos. DNA from the immunoprecipitate was subjected to qPCR analyses with primers covering the 2.1-kb region. An Fstl1 DNA genomic fragment containing no HOX binding site was used as a negative control for the locus. As shown in Fig. 4K, HOXA5 was recruited to the Fstl1 genomic region spanning the HOX binding sites whereas no binding was observed with the Fstl1 DNA negative control. Experiments done with an HOXB5 antibody did not reveal any association with the Fstl1 sequences, consistent with the lack of Hoxb5 expression in the trachea (not shown). Altogether, these data suggest that HOXA5 may act on trachea formation via in part the regulation of the transcriptional target Fstl1.
Aberrant diaphragm innervation in Hoxa5−/− embryos.
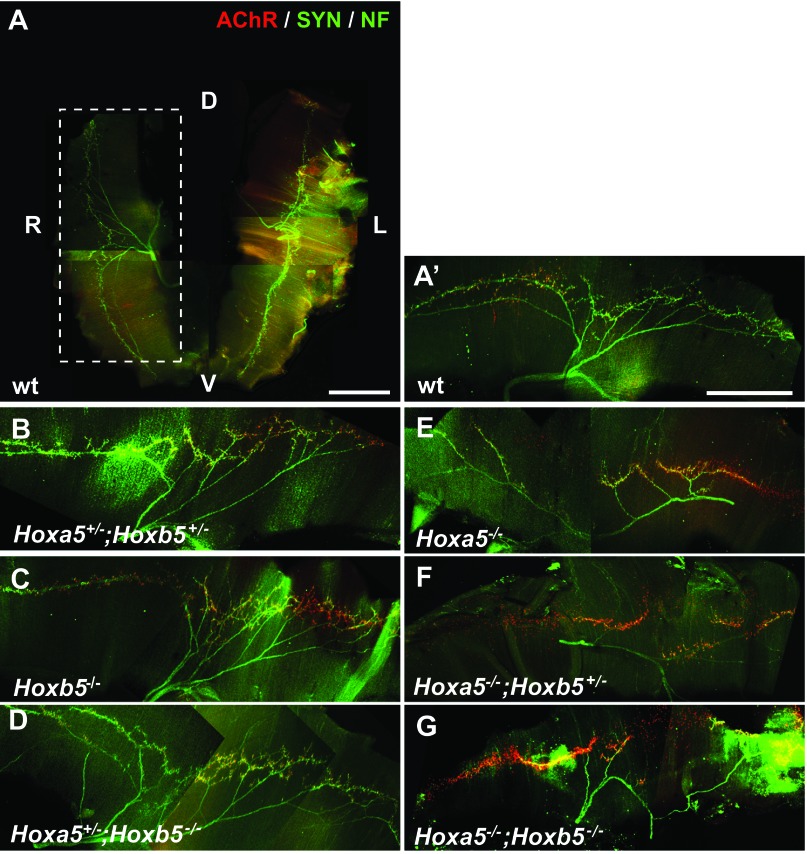
Insufficiency or lack of fetal breathing movements can participate to lung hypoplasia (25, 27, 32). Hoxa5 is expressed in motor neurons from the phrenic motor column, the latter controlling the contraction of the diaphragm (40). Motor axons from the phrenic nerve exit the cervical spinal cord to contact the diaphragmatic primordium in E12 mouse embryos. Nerve and muscle then develop together with branching and projection of the motor axons in the dorsal and ventral portions of the diaphragm (21). To assess whether the Hoxa5 and Hoxb5 mutations interfere with normal diaphragm innervation and neuromuscular synapse formation, we examined the motor axons in the diaphragm of E18.5 embryos by whole-mount IF staining with antibodies against neurofilament, synaptophysin (for presynaptic nerve terminals), and with α-bungarotoxin (for postsynaptic acetylcholine receptor). In WT, Hoxb5−/−, Hoxa5+/−;Hoxb5+/− and Hoxa5+/−;Hoxb5−/− specimens, phrenic nerves reached the diaphragm, split and projected correctly. Synapses also formed between the phrenic nerves and the diaphragm as shown by the costaining of synaptophysin and α-bungarotoxin (Fig. 5, A–D). The neuromuscular junctions were confined to the endplate band, a central region of the muscle fiber in which intramuscular nerves are located. No obvious phenotype was associated with the loss of Hoxb5 function. In contrast, Hoxa5;Hoxb5 compound mutants carrying two Hoxa5 mutant alleles presented fewer intramuscular branches and synaptic contacts (Fig. 5, E–G). There was no clear evidence that the phenotype worsened with the presence of Hoxb5 mutant alleles, suggesting that the loss of Hoxa5 function results in improper innervation of the diaphragm that may impact negatively on fetal breathing movements.
Fig. 5.
Abnormal diaphragm innervation pattern in Hoxa5;Hoxb5 embryos carrying Hoxa5 mutant alleles. A–G: analysis of diaphragm innervation patterns at E18.5 by whole-mount IF staining for neurofilament (NF), synaptophysin (SYN), and α-bungarotoxin (AChR). The dorsal-ventral (D-V) and left-right (L-R) axes are indicated on the view of the entire diaphragm (A). A–D: diaphragms from WT, Hoxb5−/−, Hoxa5+/−;Hoxb5+/−, and Hoxa5+/−;Hoxb5−/− embryos appeared normal with the phrenic nerves reaching the diaphragm correctly and synapses forming between the phrenic nerves and the diaphragm. E–G: diaphragms from Hoxa5;Hoxb5 compound mutants carrying 2 Hoxa5 mutant alleles presented fewer intramuscular branches and synaptic contacts. Scale bars: 1 mm.
Functional redundancy between Hoxa5 and Hoxb5 genes in postnatal lung development.
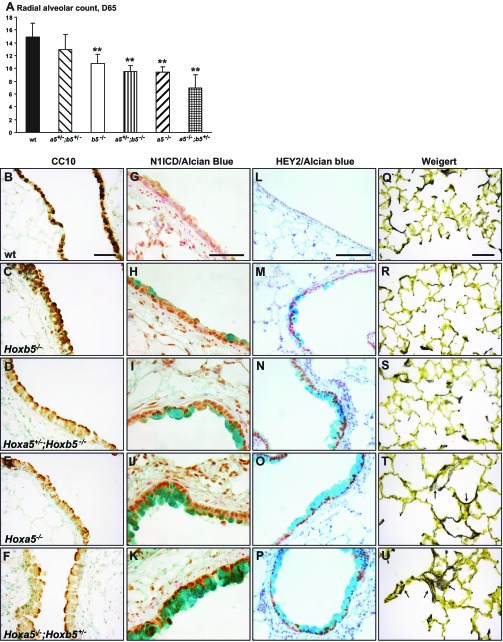
Hoxa5−/− surviving mice display lung air space enlargement and goblet cell metaplasia (33). Lung morphology of Hoxa5;Hoxb5 mutants was first investigated using a quantitative morphometric method based on radial alveolar counts. We observed decreased septa number, indicative of disruption of the alveolar growth, in Hoxa5−/− and Hoxb5−/− mutants (Fig. 6A). The phenotype worsened with the number of Hox5 mutant alleles, but again Hoxa5 appeared to play a main role in pulmonary alveogenesis.
Fig. 6.
Comparative analyses of lungs from Hoxa5;Hoxb5 adult mutant mice. A: comparison of the radial alveolar counts revealed a decreased septa number in the lungs from Hoxb5−/−, Hoxa5+/−;Hoxb5−/−, Hoxa5−/− and Hoxa5−/−;Hoxb5+/− mutants. Values are expressed as means ± SD. **P < 0.01. B–F: detection of club cells by CC10-immunostaining in lungs from Hoxa5;Hoxb5 adult mice. Decreased CC10-staining was seen in Hoxb5−/−, Hoxa5+/−;Hoxb5−/−, Hoxa5−/−, and Hoxa5−/−;Hoxb5+/− specimens. G–P: increased Notch signaling in goblet cell metaplasia areas was revealed by immunostaining for N1ICD (G–K) and HEY2 (L–P) in Hoxb5−/−, Hoxa5+/−;Hoxb5−/−, Hoxa5−/−, and Hoxa5−/−;Hoxb5+/− specimens. Q–U: Weigert staining was used for visualization of elastic fibers. In WT, Hoxb5−/− and Hoxa5+/−;Hoxb5−/− specimens, elastic fibers appeared well-distributed along the alveoli and at the tips of septa. In contrast, lungs from Hoxa5−/− and Hoxa5−/−;Hoxb5+/− mutant mice displayed disorganized and fragmented elastin aggregates within the saccular walls (arrows). Scale bars: 50 μm.
In Hoxa5−/− mice, goblet cell metaplasia originates from club to goblet cell transdifferentiation and is associated with decreased CC10 expression (7). We assessed whether the phenotype was exacerbated in Hoxa5;Hoxb5 compound mutants by evaluating club cell integrity and goblet cell metaplasia on sections of upper airways from adult mice stained, respectively, with CC10 antibody and Alcian blue for mucus detection. In WT specimens, as expected, an intense CC10 staining was detected along the bronchial airway epithelium while Alcian blue-positive cells were rarely seen (Fig. 6, B and G). In Hoxb5−/− mice, CC10 staining was strong but few goblet cell metaplasia areas were detected in upper airways (Fig. 6, C–H). A weaker CC10 labeling was observed in Hoxa5+/−;Hoxb5−/−, Hoxa5−/− and Hoxa5−/−;Hoxb5+/− specimens concomitant with the presence of Alcian blue-positive cells (Fig. 6, D–F, I–K). The phenotype worsened with the number of Hox5 mutant alleles and Hoxa5 appeared to play a key function in the specification of airway secretory cell lineages.
The molecular mechanisms underlying goblet cell metaplasia in Hoxa5−/− mice are independent of FOXA2, a transcription factor known to govern lung epithelial cell differentiation (7). Similarly, goblet cell metaplasia in lungs from Hoxb5−/− mice and Hoxa5;Hoxb5 compound mutants did not impact on FOXA2 expression when tested by immunostaining (not shown).
Notch signaling plays a key role in controlling the delicate balance between airway epithelial cell fates (23, 37, 50). As well, specific activation of the Notch pathway in airway epithelium of Hoxa5−/− mice was observed with HEY2 as a potential Notch transcriptional effector of goblet cell metaplasia (7). We evaluated the functional status of the Notch signaling pathway in airways from Hoxa5;Hoxb5 mutants by performing IHC experiments for the active intracellular domain of NOTCH1 receptor (N1ICD) and HEY2. Expression of both proteins was markedly increased at sites of goblet cell metaplasia in Hoxb5−/− and compound mutants (Fig. 6, G–P). Thus the loss of Hoxa5 function and to a lower extent the Hoxb5 mutation induce club to goblet cell transdifferentiation, a FOXA2-independent process accompanied by an increased activity of Notch signaling.
Correct formation and deposition of elastic fibers are essential for lung alveogenesis (57). Using Weigert staining to visualize the spatial distribution of elastic fibers, we observed that WT, Hoxb5−/−, and Hoxa5+/−;Hoxb5−/− mutant mice displayed focal elastic fiber deposits localized primarily at the tips of the septa, indicating no major impact of the Hoxb5 mutation on elastic fibers production (Fig. 6, Q–S). In contrast, in compound mutants carrying two Hoxa5 mutant alleles, aggregates of elastic fibers occurred within the saccular walls rather than being focused at the tips of secondary septa. They also appeared disorganized and fragmented (Fig. 6, T and U). Altogether, the results suggest that both Hoxa5 and Hoxb5 are implicated in correct lung morphology and goblet cell specification with Hoxa5 having a predominant role.
DISCUSSION
Hoxb5 is involved in lung morphogenesis.
Despite the clear presence of Hox gene expression in the developing lung, Hox function during lung morphogenesis remains largely unknown since only Hoxa5−/− mice exhibit primary lung defects leading to respiratory distress and to a high rate of lethality at birth (4, 26). In the Hox5 paralog group, Hoxa5 and Hoxb5, but not Hoxc5, are expressed along the developing respiratory tract, Hoxa5 having the broader expression domain. However, we cannot rule out the possibility of a later onset for Hoxc5 expression and some impact on lung development. During the characterization of the Hoxa5;Hoxb5 compound mutant mice, we have uncovered the role of Hoxb5 during lung development, unveiled new roles for Hoxa5 in this process and demonstrated the partial functional overlap between Hoxa5 and Hoxb5 genes.
Like Hoxa5, Hoxb5 is involved in branching morphogenesis as revealed by the decreased number of lung epithelial tubules in Hoxb5−/− embryos. This finding agrees with those of Volpe et al. (54), who reported that Hoxb5 siRNA-treated whole lung cultures showed decreased branching through the downregulation of tenascin-C, an extracellular matrix component required for branching morphogenesis. Decreased expression of tenascin-C following Hoxb5 inhibition was also shown in cultured mouse lung fibroblasts (46).
As reported in Hoxa5−/− mice, an increased number of goblet cells and air space enlargement were observed in Hoxb5−/− adult mice. Similar molecular mechanisms appeared to drive goblet cell metaplasia in Hoxb5−/− mice with no change in FOXA2 expression, and an increased Notch signaling activity in areas of goblet cell metaplasia, suggesting that both proteins act via identical means during the process of secretory epithelial cell fate in upper airways (7).
Thus the loss of Hoxb5 function impacts on lung development. The more Hoxb5 alleles are knocked out in the Hoxa5 mutant environment, the worse the lung phenotype is. Hoxb5−/− lung phenotypes are shared by Hoxa5−/− mice, indicating some functional redundancy. However, the Hoxb5 lung phenotypic traits are less severe than those encountered in Hoxa5−/− mice, suggesting that Hoxb5 is less prevalent in lung morphogenesis. This may be explained by Hoxa5 compensation in Hoxb5 mutants, as pointed out by the increased Hoxa5 expression in Hoxb5−/− lungs.
Extensive contribution of Hoxa5 to the developing respiratory system.
Pulmonary hypoplasia was observed in both Hoxa5 and Hoxb5 mutant mice and the phenotype was dramatically exacerbated in Hoxa5−/−;Hoxb5−/− embryos. Pulmonary hypoplasia is described as wet lung weight to body weight ratio inferior to 67% of normal at the time of birth (47). In E18.5 Hoxa5−/−;Hoxb5−/− embryos, the ratio was ∼50% of that of controls. Thus pulmonary hypolasia likely contributes to Hoxa5−/−;Hoxb5−/− newborn death. In Hoxa5;Hoxb5 mutants, pulmonary hypoplasia can be explained by reduced branching due to the Hoxa5 and Hoxb5 mutations and decreased proliferation in specimens carrying two Hoxa5 mutant alleles, demonstrating the specific role of Hoxa5 in governing lung growth.
Mechanical forces can influence fetal lung development through pulmonary distension (30). Deficient fetal breathing movements produced by rhythmic contraction of the respiratory muscles is a major cause of pulmonary hypoplasia and lung immaturity (25, 32). Since Hox genes are involved in motor neuron identity and connectivity, requirement for Hoxa5 and Hoxb5 in diaphragm innervation was investigated (12, 13). We observed that branching and projection of the phrenic nerve were altered and that diaphragm innervation was abnormal in specimens carrying two Hoxa5 mutant alleles, suggesting a specific role for Hoxa5 in the control of axon guidance to diaphragm. The lack of such phenotype in Hoxb5 mutants correlates with the lack of Hoxb5 expression in the phrenic motor column (40). Moreover, even though the innervation of the diaphragm was altered, the diaphragm muscle appeared intact across the abdominal wall and no visceral herniation was detected. Since spinal cord or phrenic nerve transection abolishes fetal breathing resulting in secondary lung hypoplasia, the abnormal innervation of the diaphragm specifically detected in Hoxa5−/− can contribute to the pulmonary phenotype (16, 58).
The transition from a fluid-filled lung to a functional gas-exchanging organ at birth requires the expansion of the alveolar space and a thinning of the blood-gas barrier. The distal epithelium is comprised of two specialized cell populations: type II pneumocytes, which produce surfactant for the maintenance of alveolar surface tension, and type I pneumocytes, closely associated with vascular endothelial cells for optimal gas exchanges. Interestingly, staining for T1α and PECAM was markedly reduced in Hoxa5−/− mice but no difference was detected in Hoxb5−/− mice, suggesting that Hoxb5 is not likely to have a significant role in alveolar epithelial cell and endothelial cell differentiation. In support of this, the endothelial differentiation defect was not exacerbated in Hoxa5/Hoxb5 double mutants. Thus the lack of functional type I cells in Hoxa5−/− mutants combined with the defective capillary growth might contribute to the reduction of the gas exchange surface area to a level close to that required for survival of newborns.
In situ hybridization experiments clearly revealed that among the Hox5 paralogous genes, only Hoxa5 is expressed in the mesenchyme surrounding the developing trachea. Consistent with this, the tracheal defects seen in Hoxa5 mutant mice were not observed in Hoxb5−/− mice and not amplified in Hoxa5;Hoxb5 double mutants. Similarities in tracheal cartilage defects between Hoxa5−/− and Fstl1−/− mice adding to the fact that both are confined to the mesenchymal component of the developing lung suggested that Fstl1 might be a transcriptional target of HOXA5 (20). Expression of the BMP antagonist Fstl1 was downregulated in Hoxa5−/− trachea, and ChIP experiments support the hypothesis that HOXA5 may control trachea formation through regulation of the BMP signaling pathway.
Functional predominance of Hoxa5 in lung development.
Hoxb5−/− mice survived while Hoxa5−/− mice displayed reduced viability at birth. However, the loss of both Hoxa5 and Hoxb5 gene functions caused the death at birth of all Hoxa5−/−;Hoxb5−/− pups underlying an aggravated pulmonary phenotype compared with that previously described in Hoxa5−/− mice. Characterization of compound mutants from Hox paralog groups has led to the discovery of new functions for Hox genes. For instance, Hox11 single mutants do not have kidney abnormalities, whereas Hoxa11;Hoxd11 double or Hoxa11;Hoxc11;Hoxd11 triple mutants present kidney hypoplasia and kidney agenesis, respectively, revealing the convergent role of all Hox11 paralog genes in kidney organogenesis (39, 56). Our findings on Hox5 gene action in lung development somewhat differ from those reported for the role of Hox11 genes in kidney formation since both Hoxa5 and Hoxb5 single mutants present phenotypes. However, the severity of the lung phenotype increases with the number of mutant alleles, the deletion of the Hoxa5 allele being more deleterious.
Hoxa5 can compensate for the Hoxb5 lung phenotype but the opposite is not true. This nonreciprocal compensation and the predominant Hoxa5 role may have several explanations not mutually exclusive. The mouse HOXA5 protein is 60% identical and 70% similar to HOXB5, with the homeobox and the hexapeptide sequences being 100 and 86% identical, respectively. Possibilities for the distinctive roles of Hoxa5 and Hoxb5 genes include qualitative and quantitative differences in expression as well as variations in the regulation of downstream target genes due to variable affinities in DNA binding specificity or to difference in interactions with specific cofactors.
Taken together, our results indicate that Hoxa5;Hoxb5 compound mutants exhibit synergistic lung abnormalities underscoring that both genes coordinate lung development and size. By examining the lung phenotype of Hoxa5;Hoxb5 specimens, we have unveiled Hoxb5 functions but most importantly confirmed the hierarchical importance of Hoxa5. In addition to shared functions with Hoxb5, Hoxa5 plays specific roles in lung proliferation, specification of type I pneumocytes, lung microvascular development, elastic fiber deposition, trachea formation, and diaphragm innervation. Although the specific expression of Hoxa5 in the trachea and the phrenic motor column likely underlies the trachea and the diaphragm phenotypes, an explanation for the predominant role of Hoxa5 in the lung remains to be found.
GRANTS
This work was supported by a grant from the Canadian Institutes of Health Research (MOP-15139, to L. Jeannotte), by a National Institute of Neurological Disorders and Stroke grant (R01-NS062822; to J. S. Dasen), and by a postdoctoral fellowship from the Fonds de la Recherche en Santé du Québec-Institut National de la Santé et de la Recherche Médicale (to O. Boucherat).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
O.B. and L.J. conception and design of research; O.B., S.M., F.-A.B.-S., J.A., P.P., and D.M.W. performed experiments; O.B., S.M., F.-A.B.-S., J.A., P.P., and L.J. analyzed data; O.B. and L.J. interpreted results of experiments; O.B. prepared figures; O.B. drafted manuscript; J.S.D. and L.J. edited and revised manuscript; L.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. J. Charron for helpful comments on the manuscript, Drs. A. Boulet and M. Capecchi for collaboration in obtaining the Hoxb5 mutant mouse line, Drs. B. Hogan and P. Sharpe for in situ probes, Dr. G. Singh for antibody, Dr. J. Côté for advices for ChIP experiments, and M. Lemieux for technical assistance.
REFERENCES
- 1. Aubin J, Chailler P, Ménard D, Jeannotte L. Loss of Hoxa5 gene function in mice perturbs intestinal maturation. Am J Physiol Cell Physiol 277: C965–C973, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Aubin J, Déry U, Lemieux M, Chailler P, Jeannotte L. Stomach regional specification requires Hoxa5-driven mesenchymal-epithelial signaling. Development 129: 4075–4087, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Aubin J, Lemieux M, Tremblay M, Behringer RR, Jeannotte L. Transcriptional interferences at the Hoxa4/Hoxa5 locus: importance of correct Hoxa5 expression for the proper specification of the axial skeleton. Dev Dyn 212: 141–156, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Aubin J, Lemieux M, Tremblay M, Bérard J, Jeannotte L. Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract defects. Dev Biol 192: 432–445, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Bogue CW, Gross I, Vasavada H, Dynia DW, Wilson CM, Jacobs HC. Identification of Hox genes in newborn lung and effects of gestational age and retinoic acid on their expression. Am J Physiol Lung Cell Mol Physiol 266: L448–L454, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Bogue CW, Lou LJ, Vasavada H, Wilson CM, Jacobs HC. Expression of Hoxb genes in the developing mouse foregut and lung. Am J Respir Cell Mol Biol 15: 163–171, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Boucherat O, Chakir J, Jeannotte L. The loss of Hoxa5 function in mice promotes NOTCH-dependent goblet cell metaplasia in lung airways. Biol Open 1: 677–691, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boulet AM, Capecchi MR. Targeted disruption of hoxc-4 causes esophageal defects and vertebral transformations. Dev Biol 177: 232–249, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Colvin JS, White AC, Pratt SJ, Ornitz DM. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development 128: 2095–2106, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Condie BG, Capecchi MR. Mice with targeted disruptions in the paralogous genes hoxa-3 and hoxd-3 reveal synergistic interactions. Nature 370: 304–307, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Coulombe Y, Lemieux M, Moreau J, Aubin J, Joksimovic M, Bérubé-Simard FA, Tabariès S, Boucherat O, Guillou F, Larochelle C, Tuggle CK, Jeannotte L. Multiple promoters and alternative splicing: Hoxa5 transcriptional complexity in the mouse embryo. PLoS One 5: e10600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dasen JS, Jessell TM. Hox networks and the origins of motor neuron diversity. Curr Top Dev Biol 88: 169–200, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell 123: 477–491, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Del Moral PM, Sala FG, Tefft D, Shi W, Keshet E, Bellusci S, Warburton D. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol 290: 177–188, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Emery JL, Mithal A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child 35: 544–547, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fewell JE, Lee CC, Kitterman JA. Effects of phrenic nerve section on the respiratory system of fetal lambs. J Appl Physiol 51: 293–297, 1981 [DOI] [PubMed] [Google Scholar]
- 17. Garin E, Lemieux M, Coulombe Y, Robinson GW, Jeannotte L. Stromal Hoxa5 function controls the growth and differentiation of mammary alveolar epithelium. Dev Dyn 235: 1858–1871, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Gaunt SJ, Coletta PL, Pravtcheva D, Sharpe PT. Mouse Hox-3.4: homeobox sequence and embryonic expression patterns compared with other members of the Hox gene network. Development 109: 329–339, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Gendronneau G, Boucherat O, Aubin J, Lemieux M, Jeannotte L. The loss of Hoxa5 function causes estrous acyclicity and ovarian epithelial inclusion cysts. Endocrinology 153: 1484–1497, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, Qiao L, Geng H, Nakajima M, Furuichi T, Ikegawa S, Gao X, Chen YG, Jiang D, Ning W. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci USA 108: 7058–7063, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greer JJ, Allan DW, Martin-Caraballo M, Lemke RP. An overview of phrenic nerve and diaphragm muscle development in the perinatal rat. J Appl Physiol 86: 779–786, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Greer JM, Puetz J, Thomas KR, Capecchi MR. Maintenance of functional equivalence during paralogous Hox gene evolution. Nature 403: 661–665, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development 136: 1751–1759, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hallock PT, Xu CF, Park TJ, Neubert TA, Curran T, Burden SJ. Dok-7 regulates neuromuscular synapse formation by recruiting Crk and Crk-L. Genes Dev 24: 2451–2461, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harding R, Hooper SB, Han VK. Abolition of fetal breathing movements by spinal cord transection leads to reductions in fetal lung liquid volume, lung growth, and IGF-II gene expression. Pediatr Res 34: 148–153, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Herriges JC, Yi L, Hines EA, Harvey JF, Xu G, Gray PA, Ma Q, Sun X. Genome-scale study of transcription factor expression in the branching mouse lung. Dev Dyn 241: 1432–1453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inanlou MR, Baguma-Nibasheka M, Kablar B. The role of fetal breathing-like movements in lung organogenesis. Histol Histopathol 20: 1261–1266, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Jaffe L, Jeannotte L, Bikoff EK, Robertson EJ. Analysis of beta 2-microglobulin gene expression in the developing mouse embryo and placenta. J Immunol 145: 3474–3482, 1990 [PubMed] [Google Scholar]
- 29. Jeannotte L, Lemieux M, Charron J, Poirier F, Robertson EJ. Specification of axial identity in the mouse: role of the Hoxa-5 (Hox1.3) gene. Genes Dev 7: 2085–2096, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Kitterman JA. The effects of mechanical forces on fetal lung growth. Clin Perinatol 23: 727–740, 1996 [PubMed] [Google Scholar]
- 31. Krumlauf R, Holland PW, McVey JH, Hogan BL. Developmental and spatial patterns of expression of the mouse homeobox gene, Hox 2.1. Development 99: 603–617, 1987 [DOI] [PubMed] [Google Scholar]
- 32. Liggins GC, Vilos GA, Campos GA, Kitterman JA, Lee CH. The effect of spinal cord transection on lung development in fetal sheep. J Dev Physiol 3: 267–274, 1981 [PubMed] [Google Scholar]
- 33. Mandeville I, Aubin J, LeBlanc M, Lalancette-Hébert M, Janelle MF, Tremblay GM, Jeannotte L. Impact of the loss of Hoxa5 function on lung alveogenesis. Am J Pathol 169: 1312–1327, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell 68: 283–302, 1992 [DOI] [PubMed] [Google Scholar]
- 35. McIntyre DC, Rakshit S, Yallowitz AR, Loken L, Jeannotte L, Capecchi MR, Wellik DM. Hox patterning of the vertebrate rib cage. Development 134: 2981–2989, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Meunier D, Aubin J, Jeannotte L. Perturbed thyroid morphology and transient hypothyroidism symptoms in Hoxa5 mutant mice. Dev Dyn 227: 367–378, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J Cell Sci 123: 213–224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 18: 8–23, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patterson LT, Pembaur M, Potter SS. Hoxa11 and Hoxd11 regulate branching morphogenesis of the ureteric bud in the developing kidney. Development 128: 2153–2161, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Philippidou P, Walsh C, Aubin J, Jeannotte L, Dasen JS. Sustained Hox5 gene activity is required for respiratory motor neuron development. Nat Neurosci 15: 1636–1644, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Potvin E, Beuret L, Cadrin-Girard JF, Carter M, Roy S, Tremblay M, Charron J. Cooperative action of multiple cis-acting elements is required for N-myc expression in branchial arches: specific contribution of GATA3. Mol Cell Biol 30: 5348–5363, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramirez MI, Millien G, Hinds A, Cao Y, Seldin DC, Williams MC. T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev Biol 256: 61–72, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Rancourt DE, Tsuzuki T, Capecchi MR. Genetic interaction between hoxb-5 and hoxb-6 is revealed by nonallelic noncomplementation. Genes Dev 9: 108–122, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Rijli FM, Chambon P. Genetic interactions of Hox genes in limb development: learning from compound mutants. Curr Opin Genet Dev 7: 481–487, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Rossel M, Capecchi MR. Mice mutant for both Hoxa1 and Hoxb1 show extensive remodeling of the hindbrain and defects in craniofacial development. Development 126: 5027–5040, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Roth-Kleiner M, Hirsch E, Schittny JC. Fetal lungs of tenascin-C-deficient mice grow well, but branch poorly in organ culture. Am J Respir Cell Mol Biol 30: 360–366, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Seegmiller RE, Cooper CA, Houghton MJ, Carey JC. Pulmonary hypoplasia in chondrodystrophic mice. Teratology 33: 339–347, 1986 [DOI] [PubMed] [Google Scholar]
- 48. Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn 212: 482–494, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Sylva M, Li VSW, Buffing AAA, van Es JH, van den Born M, van der Velden S, Gunst Q, Koolstra JH, Moorman AFM, Clevers H, van den Hoff MJB. The BMP antagonist follistatin-like 1 is required for skeletal and lung organogenesis. PLoS ONE 6: e22616, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136: 2297–2307, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tvrdik P, Capecchi MR. Reversal of Hox1 gene subfunctionalization in the mouse. Dev Cell 11: 239–250, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Volpe MV, Archavachotikul K, Bhan I, Lessin MS, Nielsen HC. Association of bronchopulmonary sequestration with expression of the homeobox protein Hoxb-5. J Pediatr Surg 35: 1817–1819, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Volpe MV, Pham L, Lessin M, Ralston SJ, Bhan I, Cutz E, Nielsen HC. Expression of Hoxb-5 during human lung development and in congenital lung malformations. Birth Defects Res A Clin Mol Teratol 67: 550–556, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Volpe MV, Ramadurai SM, Pham LD, Nielsen HC. Hoxb-5 down regulation alters Tenascin-C, FGF10 and Hoxb gene expression patterns in pseudoglandular period fetal mouse lung. Front Biosci 12: 860–873, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR, Jesudason E. Lung organogenesis. Curr Top Dev Biol 90: 73–158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wellik DM, Hawkes PJ, Capecchi MR. Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev 16: 1423–1432, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol 23: 320–326, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Wigglesworth JS, Desai R. Effect on lung growth of cervical cord section in the rabbit fetus. Early Hum Dev 3: 51–65, 1979 [DOI] [PubMed] [Google Scholar]