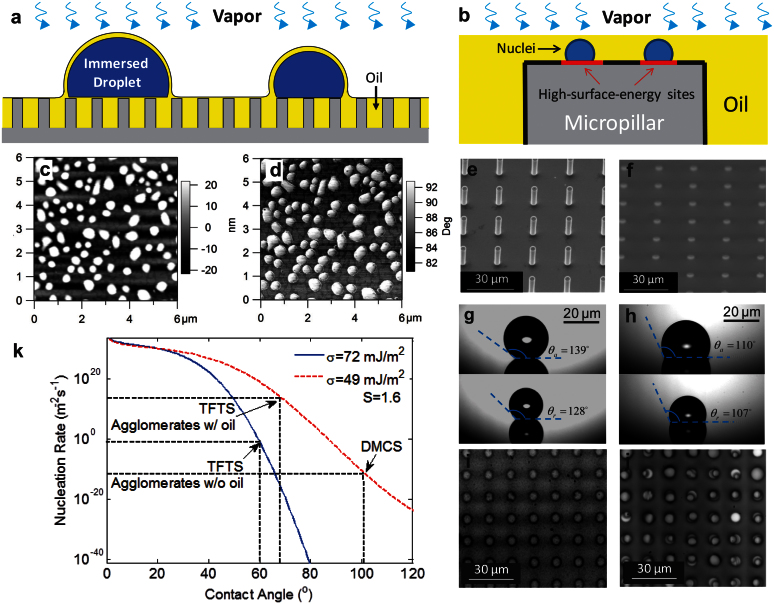
Figure 2. Mechanism of immersion condensation.
(a) Schematic showing water vapor diffusing through the thin oil film and forming immersed droplets on the tips of micropillars. (b) Magnified schematic showing the nuclei formation on high-surface-energy sites on micropillar tips in the oil. (c) and (d) Height and phase images of atomic force microscope (AFM) images of TFTS ((Tridecafluoro-1,1,2,2-tetrahydrooctyl)-1-trichlorosilane) coating. The higher phase angle at the nanoagglomerates indicates local higher surface energy. (e) and (f) Environmental scanning electron microscope (ESEM) images of TFTS-coated micropillar arrays before and after the oil-infusion. (g) and (h) Contact angle hysteresis on a superhydrophobic surface without and with oil-infusion. The hysteresis is ≈3° on the oil-infused surface with a contact angle ≈110°. The microstructure geometries were the same on both surfaces, with diameter of 5 μm, height of 20 μm, and period of 15 μm. (i) and (j) White-light optical microscope images of condensation on micropillar arrays before and after oil-infusion. The micropillar geometries were the same as (g) and (h). The supersaturation in the experiments was S = 1.6. (k) Nucleation rates predicted as a function of contact angle and interfacial energy.