Fig. 1.
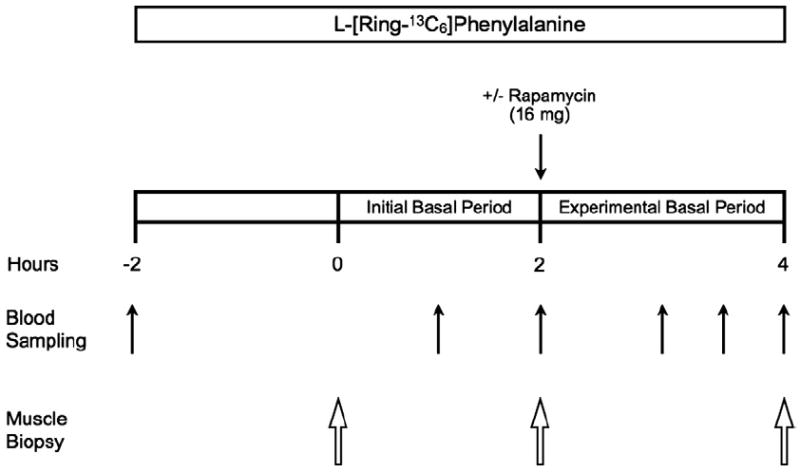
Schematic of the experimental trial. The experimental trial was designed to obtain measurements during two consecutive 2h basal post-absorptive periods, referred to as the initial basal period and experimental basal period, respectively. Each subject completed the experimental trial on two occasions (Control and Rapamycin) in a randomized, counterbalanced cross-over fashion. The experimental trial was identical for the Control and Rapamycin trials except that during one experimental trial subjects ingested 16mg of rapamycin (Rapamycin trial) at the start of the experimental basal period, whereas no rapamycin was ingested during the other experimental trial (Control trial).
