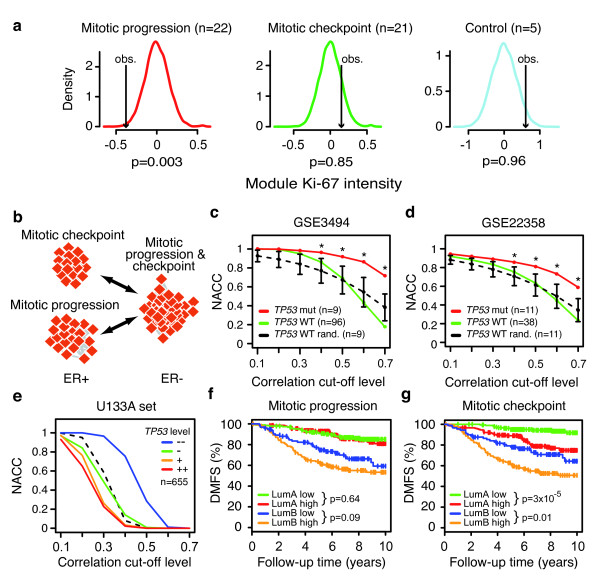
Figure 2.
Separation of cell cycle genes into two modules is dependent on TP53 status. (a) Module genes were assayed for effects on proliferation in the KPL-4 breast cancer cell line using an RNAi-based cell spot microarray system. Knockdown of genes in the mitotic progression module significantly inhibited cell proliferation as assayed using Ki-67 staining intensity (P = 0.003, left panel), whereas knockdown of genes in the mitotic checkpoint module did not show any significant effects (P = 0.85, center panel). A group of non-specific control siRNAs showed that the majority of genes in the assayed siRNA library abrogate cellular proliferation (right panel). Module effects on KPL-4 proliferation was estimated by comparing the observed mean Ki-67 intensity for the module genes (black arrows) and compared to background Ki-67 distributions (density curves) based on 10,000 random groups of the same size as the assayed module. P-values are one-sided. (b) The mitotic progression and checkpoint modules are separated in ER-positive breast cancer, but interconnected as a single module in ER-negative breast cancer. (c, d) The separation of the mitotic progression and checkpoint modules relate to sample TP53 mutation status. Interconnection between the mitotic progression and checkpoint modules were assayed using the NACC at increasing cut-off correlation levels in luminal A and luminal B samples. NACC was calculated in luminal samples with known TP53 mutation status from the (c) GSE3494 and (d) GSE22358 breast cancer datasets. TP53 wildtype (WT) samples showed a clear separation between the mitotic progression and checkpoint modules at increasing correlation cut-off levels (green lines). However, in TP53-mutated samples modules remained interconnected at higher levels of correlation (red lines). The NACC for TP53-mutated samples was compared to 10,000 random selections of the same number of TP53 WT samples (black dashed lines) and stars denote permutation-based p-values below 0.05. Error bars represent standard deviations. (e) Luminal samples from the U133A set were divided into quartile groups based on TP53 expression and NACC between mitotic progression and checkpoint modules were calculated within these groups. Decreasing TP53 expression correlated to higher level of interconnection between the mitotic progression and checkpoint modules with the highest TP53 expression quartile samples showing a distinctly higher module interconnection than the lowest quartile samples. As reference the NACC for all luminal samples is shown (black dotted line). (f) Dichotomizing breast cancer patients of either luminal A (LumA) or luminal B (LumB) subtype on mitotic progression module activity did not add prognostic information (P = 0.6 and P = 0.09, log-rank tests) using DMFS as endpoint, (g) while an above mean activity of the mitotic checkpoint module identified groups within both luminal A and luminal B tumors with worse prognosis (luminal A P = 3*10-5, luminal B P = 0.01, log-rank tests).