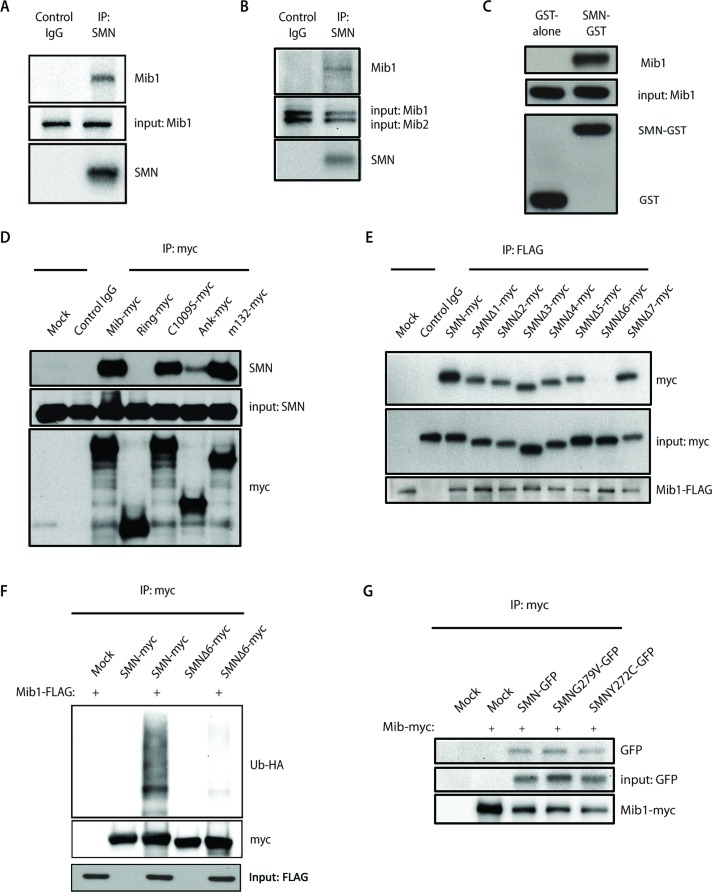
FIGURE 4:
(A) Endogenous SMN was immunoprecipitated from HEK-293T cell lysates with an antibody to SMN, and immunoprecipitates were resolved by SDS–PAGE. Association with Mib1 was determined by Western blot analysis using an antibody to Mib1. (B) Protein lysates from HEK-293T cells were immunoprecipitated with an SMN antibody. While Mib1 coimmunoprecipitated with SMN, Mib2 was not detected by Western blot analysis. Membranes were probed with anti-SMN, anti-Mib1, and anti-Mib2 antibodies. (C) GST or GST-SMN was used to pull down purified recombinant Mib1 protein. (D) Myc-tagged Mib1 constructs containing either full-length or truncated transcripts of the Mib1 gene were transiently transfected into HEK-293T cells. Immunoprecipitation using an anti-myc antibody was performed, and interaction with endogenous SMN was detected by Western blot analysis using an anti-SMN antibody. (E) HEK-293T cells were transfected with 0.5 μg full-length SMN cDNA or various SMN constructs with a single-exon deletion, and 2 μg Mib1-FLAG. Mib1-FLAG was immunoprecipitated and resolved on a SDS–PAGE gel. Western blots were probed with antibodies to FLAG and Myc to determine binding of Mib1 to SMN. (F) HEK-293T cells were transfected with 2 μg Mib1-FLAG, 1 μg HA-Ub, and 1 μg either full-length SMN-myc or SMNΔ6-myc. The cells were harvested 48 h later, myc-tagged SMN was immunoprecipitated using an anti-myc antibody, and the proteins were separated by SDS–PAGE and detected by Western blotting. The blots were probed with an HA antibody to detect ubiquitinated SMN. (G) HEK-293T cells were transfected with 2 μg Mib1-myc and either 0.5 μg GFP-tagged wild-type SMN, SMN G279V, or SMN Y272C. The cells were harvested 48 h later, and SMN was immunoprecipitated with an anti-myc antibody and resolved on a SDS–PAGE gel. Western blots were probed with antibodies to myc and GFP to determine interaction of Mib1 and wild-type SMN or the disease-associated mutants.