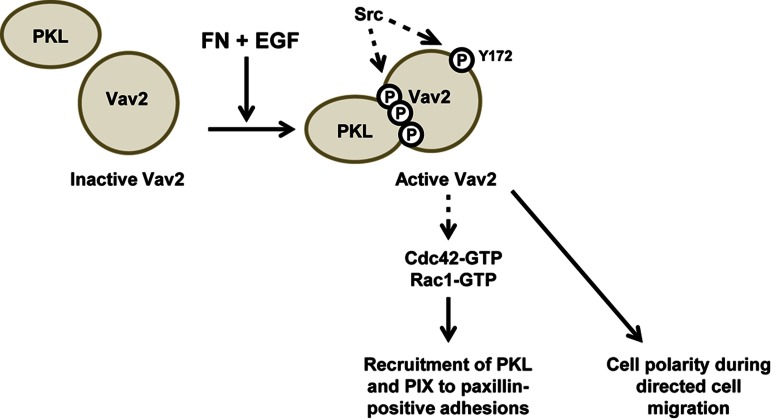
FIGURE 10:
Schematic representation of findings outlined in this study. Phosphorylated PKL binds Vav2 via the Vav2 SH2 domain in response to EGF and FN signaling, resulting in increased Vav2 phosphorylation and stimulation of its GEF activity. These phosphorylation events are likely mediated by Src tyrosine kinase activity (data not shown; Servitja et al., 2003; Tu et al., 2003; Brown et al., 2005; Yu et al., 2009)). Active Vav2 promotes increased recruitment of PKL and β-PIX to paxillin-positive adhesions in an active Cdc42- and Rac1-dependent manner. In addition, Vav2 is required for efficient polarization of cells during directed cell migration and the corresponding distribution of PKL and β-PIX to the leading edge. In addition to the data outlined here, PKL may also facilitate the switching of Vav2 activity toward RhoA at later spreading time points and during migration to regulate adhesion maturation and disassembly.