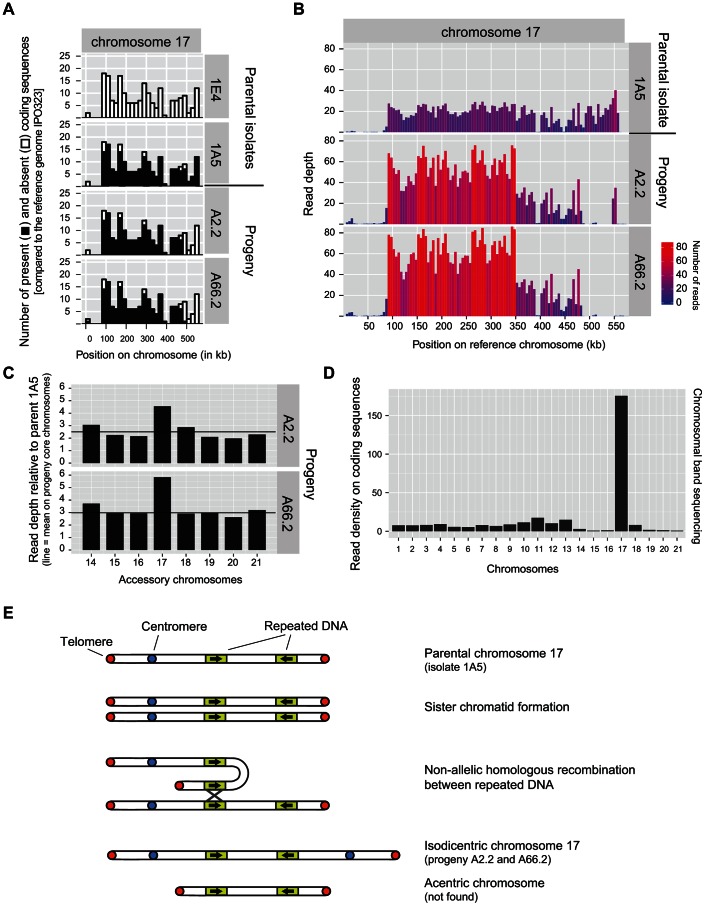
Figure 10. Initiation of a breakage-fusion-bridge cycle of Zymoseptoria tritici chromosome 17 during meiosis.
A) Genome resequencing of two progeny (A2.2 and A66.2) from a cross between the parental isolates 1E4 and 1A5. Parent 1A5 carries accessory chromosome 17 (parent 1E4 is missing chromosome 17). Illumina sequencing reads were mapped to known coding regions of chromosome 17 on the reference genome IPO323. Black and white segments of the bars represent presence and absence, respectively, of particular coding sequences in 20 kb sections along chromosomes 17. B) Variation in read density along chromosome 17 of parental isolate 1A5 and progeny A2.2 and A66.2. C) Variation in read density among accessory chromosomes in the offspring A2.2 and A66.2. Illumina sequencing reads were mapped to coding sequences of the reference genome IPO323. Read density is reported as fold-difference between the offspring and the parental isolate 1A5. As a reference, the mean fold-difference on core chromosomes is reported as a horizontal line. Both offspring showed a near two-fold higher read density on chromosome 17 compared to other accessory chromosomes. D) Illumina sequencing of an excised chromosomal band at 0.9 Mb identified by PFGE in the offspring A2.2. Illumina sequencing reads were mapped to coding sequences of all chromosomes of the reference genome IPO323. E) Schematic illustration of the hypothesized non-allelic homologous recombination between inverted repeats that generated chromosome 17 in offspring A2.2 and A66.2. The resulting isodicentric chromosome can initiate a breakage-fusion-bridge cycle while the acentric chromosome will be lost during successive rounds of cell division.