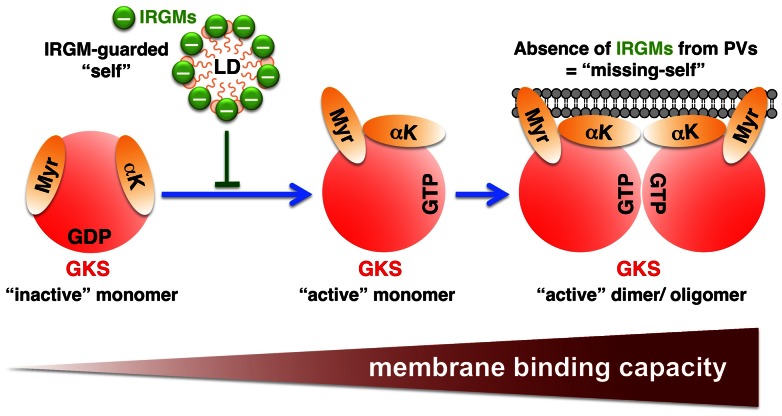
Figure 11. Proposed model: IRGM proteins regulate the localization of GKS and GBP proteins away from “self” membranes and towards “non-self” PVs.
Upon GTP acquisition ( = “activation”), GKS and GBP proteins transition into a conformational state that permits lipid binding. Protein oligomerization of activated GKS and GBP proteins further increases their membrane-binding activity and is critical for the stable association of GKS and GBP proteins with most membranes. IRGM proteins negatively regulate the “activation” and subsequent oligomerization of GKS and GBP proteins. Accordingly, IRGM-positive membranes/micelles (e.g. LDs) are protected against GKS and GBP binding and IRGM-free membranes (e.g. surrounding PVs) are targets for GKS and GBP binding. In addition to protecting self-structures, IRGM proteins also maintain a pool of inactive, monomeric GKS and GBP proteins. Monomeric GKS and GBP proteins are cytosolic and move around inside the cell until they encounter PVs. In Irgm1/m3−/− cells, the binding to IRGM-deficient endomembranes decreases the pool of available, monomeric GKS and GBP proteins and therefore diminishes the targeting of GKS and GBP proteins to PVs. In this illustration, the GKS protein Irgb10 with its two the putative lipid binding domains is given as an example (Myr = myristoyl group; αK = amphipathic helix).