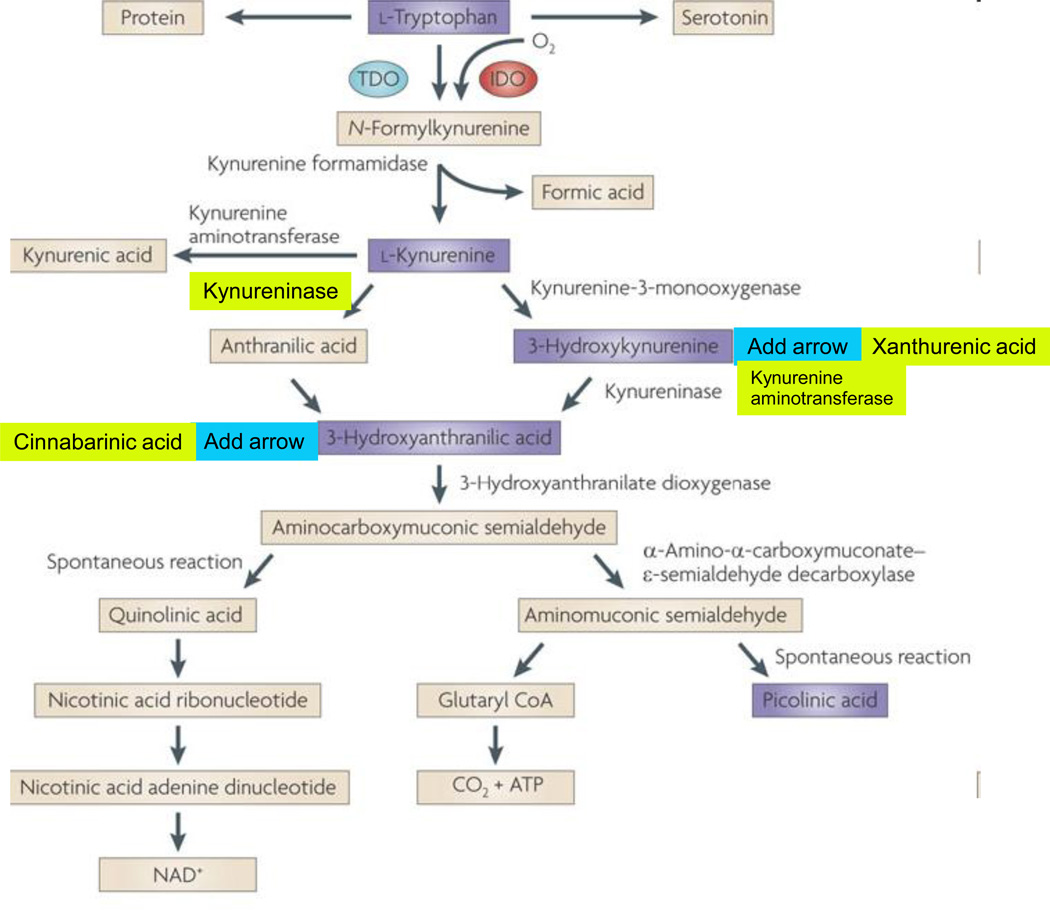
Figure 1. The kynurenine pathway of tryptophan degradation in mammals.
The kynurenine pathway is initiated by the transformation of tryptophan to N-formylkynurenine by indoleamine 2,3-dioxygenases (IDO) 1 186 and 2 187, and tryptophan 2,3-dioxygenase (TDO) 188. N-formylkynurenine is degraded by formamidase to yield the pivotal metabolite kynurenine. Compared to peripheral organs, the activities of these enzymes in the brain are normally low. Kynurenine undergoes irreversible transamination to form kynurenic acid (KYNA). Four kynurenine aminotransferases (KATs) have so far been shown to catalyze this reaction 189. In the mammalian brain, KAT II is thought to be the main biosynthetic enzyme of KYNA as it does not recognize competing abundant amino acids as substrates 190. No degradative enzyme of KYNA has yet been described in the mammalian brain.
A second branch of the kynurenine pathway, competing with KYNA synthesis by KATs, is initiated by kynurenine 3-monooxygenase (KMO) and kynureninase, which catalyze the degradation of kynurenine to 3-hydroxykynurenine (3-HK) and anthranilic acid, respectively 177. The in vivo disposition of kynurenine in the brain depends on the cellular localization, intracellular compartmentalization and kinetic characteristics of these two degradative enzymes, as well as KATs 41, 57. Like kynurenine, 3-HK serves as a substrate of competing enzymes: kynureninase and KAT II. Mammalian kynureninase preferentially recognizes 3-HK over kynurenine, catalyzing the formation of 3-HANA 191. Compared to peripheral organs and for unknown reasons, anthranilic acid is a far better precursor of 3-HANA than 3-HK within the brain 30. KAT II, and possibly other KATs, convert 3-HK to xanthurenic acid, which, like KYNA, appears to constitute a dead-end product of the kynurenine pathway.
In addition to being oxidized non-enzymatically to cinnabarinic acid, 3-HANA is the substrate of 3-hydroxyanthranilic acid 3,4-dioxygenase (3HAO), which is present in relative abundance in the brain 192, forming α-amino-α-carboxymuconic-ω-semialdehyde. The kynurenine pathway branches then branches into two arms. Under physiological conditions, α-amino-α-carboxymuconic-ω-semialdehyde spontaneously rearranges to form QUIN with a half-life of approximately 20 minutes. QUIN synthesis may therefore be regulated both at the level of 3HAO (for example by Fe2+ 193) and by changes in the intracellular milieu (pH, temperature) to influence the second, non-enzymatic step. Notably, the brain seems to contain very little α-amino-α-carboxymuconic-ω-semialdehyde carboxylase, an enzyme which deflects the metabolic cascade towards the production of picolinic and glutaric acids 194. The cerebral activity of QUIN's degradative enzyme, quinolinate phosphoribosyltransferase, is very low 195, making this enzyme one of the gatekeepers for the synthesis of NAD+ 196–197.