Abstract
Background
Candida is the third most common cause of late-onset neonatal sepsis in infants born at < 1500 g. C. parapsilosis infections are increasingly reported in preterm neonates in association with indwelling catheters.
Methods
We systematically reviewed neonatal literature and synthesized data pertaining to percentage of C. parapsilosis infections and mortality by meta-analyses. We also reviewed risk factors, virulence determinants, antimicrobial susceptibility patterns and outlined clinical management strategies.
Results
C. parapsilosis infections comprised 33.47 % [95% CI, 30.02, 37.31] of all neonatal Candida infections. C. parapsilosis rates were similar in studies performed before the year 2000, 33.53 % [95% CI, 30.06, 37.40] (28 studies), to those after 2000, 27.00% [95% CI, 8.25, 88.37] (8 studies). The mortality due to neonatal Candida parapsilosis infections was 10.02% [95% CI, 7.66, 13.12]. Geographical variations in C. parapsilosis infections included a low incidence in Europe and higher incidence in North America and Australia. Biofilm formation was a significant virulence determinant and predominant risk factors for C. parapsilosis infections were prematurity, prior colonization and catheterization. Amphotericin B remains the antifungal drug of choice and combination therapy with caspofungin or other echinocandins may be considered in resistant cases.
Conclusion
C. parapsilosis is a significant neonatal pathogen, comprises a third of all Candida infections and is associated with 10% mortality. Availability of tools for genetic manipulation of this organism will identify virulence determinants and organism characteristics that may explain predilection for preterm neonates. Strategies to prevent horizontal transmission in the neonatal unit are paramount in decreasing infection rates.
Keywords: Neonate, Candida parapsilosis, systematic review, meta-analyses
Introduction
Neonatal sepsis is frequently due to organisms colonizing the skin and mucosal surfaces, such as Coagulase negative Staphylococci and Candida (1). Candida is the third most common etiologic agent in late-onset neonatal sepsis (> 72 hrs of age) and is responsible for 8 to 15% of hospital-acquired infections (2). Candida infections are responsible for an ‘attributable mortality’ of 18–25%, significant morbidity and healthcare costs (7, 30, 53). Overall, hospital infections due to non-albicans Candida are increasing and Candida parapsilosis is among the three most common Candida blood isolates (3–10). Compared to C. albicans, mortality due to C. parapsilosis infections is lower in adults (3, 4, 11), but has not been adequately evaluated in very low birth weight (VLBW, birth weight < 1500 g) neonates.
Preterm infants have high Candida colonization rates compared to term infants and it is well established that colonization with Candida is inversely proportional to gestational age (12, 13). Colonization precedes invasive Candida infection and the number of colonization sites and density of skin colonization with Candida correlate with candidemia (14–16). Premature neonates including VLBW and extremely low birth weight (ELBW, birth weight < 1000 g) infants frequently require vascular catheters for administration of parenteral nutrition to meet nutritional needs. Adherence properties of C. parapsilosis that favor adherence to the skin and catheters may be responsible for increased incidence of infection in preterm neonates. Increases in C. parapsilosis infections and their associated morbidity and mortality make this organism a significant infectious burden in VLBW preterm neonates (17). In this article, we have specifically reviewed neonatal C. parapsilosis infections with respect to organism characteristics, epidemiology, risk factors, antimicrobial susceptibility and mortality.
Clinical Epidemiology of C. parapsilosis Infections
C. parapsilosis is ubiquitous in nature and is found as a commensal on the human skin. It is most frequently isolated from hands (subungual space) and the gastrointestinal (GI) tract (18–22). The presence of C. parapsilosis on human hands may contribute to the horizontal transmission of this organism in neonatal intensive care units (21–24). Neonatal risk factors for invasive C. parapsilosis infections are birth weight < 1500 g, prematurity, prior colonization (25), parenteral nutrition, intravascular catheters and use of antibiotics, steroids and H2 blockers (3, 18, 20). Exogenous sources of infection may be important (26) but colonization of the skin, GI and the respiratory tract often precede neonatal invasive infections (16, 25). The increasing awareness of C. parapsilosis infections in neonates is exemplified by the increased number of publications related to this organism in the last 2 decades (Table. 1). We performed a systematic review and meta-analysis to discern the clinical epidemiology and mortality of neonatal infections due to C. parapsilosis. We followed published guidelines for reporting of ‘Meta-analyses of Observational Studies in Epidemiology’ wherever relevant (27). We hypothesized that C. parapsilosis is responsible for a significant proportion of neonatal Candida infections and is associated with significant mortality.
Methods of the Systematic review and Meta-analyses
We searched Pubmed from 1990 to April 2012, using the search terms ‘Candida parapsilosis’ and the root word ‘neonat*’. Our search strategy yielded 226 publications, whose citations were reviewed to identify those that included a significant neonatal component.
Inclusion criteria
Observational studies (cohort and case-control studies) or randomized trials (studies of anti-fungal agents, where data regarding the incidence of Candida infections and mortality were extractable) were included. Publications that reported 10 or more neonatal patients or neonatal clinical isolates were selected and data regarding incidence rate of C. parapsilosis infections as a component of total Candida infections and mortality were extracted by author MP (Table 2). All studies without a neonatal component (less than 10 patients or clinical isolates) or where data for neonates or C. parapsilosis could not be separately extracted were excluded.
Table 2.
Reports of neonatal C. parapsilosis infections from 1990 to 2012 with > 10 clinical patients or isolates
| Reference | Period of study | Reported | Place | Bwt | GA | No of infections | Mortality | Comments | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP | CA | OT | Total | CP | CA | All | |||||||
| Faix et al (115) | 1980–90 | 1992 | USA | 16 | 29 | 45 | 0/16 | 7/29 | 7/45 | ||||
| Shian et al (116) | 1993 | Taiwan | 2 | 16 | 18 | 10/18 | |||||||
| Saxen et al (117) | 55 mths | 1995 | Finland | Mean 817g | Mean 28 wks | 58 | Study of CP only | ||||||
| Stamos et al (118) | Jan 88–Oct 92 | 1995 | USA | Preterm infants | 4 | 9 | 3 | 16 | 65 pediatric patients | ||||
| Padovani et al (119) | 1997 | Italy | Mean 1405 g | Mean 29 wks | 2 | 23 | 1 | 26 | 11/26 | ||||
| Vazquez et al (120) | 1992–93 | 1997 | USA | Mean 890 g | Mean 26 wks | 11 | 7 | 24 | |||||
| Huttova et al (121) | Jan 93–Dec 97 | 1998 | Slovakia | Median 1290 g | Median 33.5 wks | 3 | 37 | 40 | 8/40 | ||||
| Cabellero 1998 (122) | Mar 94–Sep 97 | 1998 | Spain | 2 term and 12 preterm | 7 | 7 | 14 | 3/14 | |||||
| Kossoff et al (10) | Jan 81–Dec 95 | 1998 | USA | Median 765g | Median 26 wks | 54 | 50 | 3 | 107 | 2/54 | 13/50 | 29/107 | |
| Levy et al (123) | 1989–96 | 1998 | USA | 24 | 9 | 35 | 4/35 | Study in children 0–20 yrs of age. (80 patients) | |||||
| Khatib et al (124) | 1998 | USA | 18 | 15 | 33 | ||||||||
| Huang et al (125) | 1999 | Taiwan | Mean 1249g | Mean 29.2 wks | 17 | 0 | 17 | Study of CP only | |||||
| Benjamin et al (109) | Jan 95–Jul 98 | 2000 | USA | Mean 1178g | Mean 27.6 wks | 19 | 15 | 3 | 37 | 8/19 | 5/15 | 13/37 | |
| Karlowicz et al (99) | Jan 94–Dec 98 | 2000 | USA | Range 426–3190 g | Range 23–40 wks | 54 | 44 | 6 | 104 | 9/54 | 2/44 | 11/104 | Study of Catheter-associated Candidemia |
| Juster-Reicher et al (103) | Sept 94–Jan 98 | 2000 | Israel | Mean 847g | Mean 26 wks | 9 | 12 | 3 | 24 | 2/9 | 2/12 | 4/24 | Study of liposomal amphotericin |
| Huang et al (126) | Jan 94–Jul 97 | 2000 | Taiwan | Range 768–3700 g | Range 25–42 wks | 21 | 24 | 2 | 47 | 8/21 | 9/24 | 17/47 | |
| Krcmery et al (127) | 1989–98 | 2000 | Slovakia | 10 | 68 | 2 | 80 | 4/10 | 22/68 | 27/80 | Slovakia fungal group | ||
| Gupta et al (128) | 6 mths | 2001 | India | 1 | 4 | 14 | 19 | ||||||
| Makhoul et al (129) | Jan 89–Dec 98 | 2001 | Israel | 13 | 21 | 18 | 52 | ||||||
| Fairchild 2002 (106) | 1991–98 | 2002 | USA | 8 | 41 | 9 | 58 | ||||||
| Juster-Reicher 2003 et al (102) | Jul 95–Jan 01 | 2003 | Israel | Median 860 g | Median 26 wks | 17 | 15 | 5 | 37 | 1/37 | Study of high dose liposomal therapy | ||
| Mestroni et al (130) | Nov 98–Aug 01 | 2003 | Argentina | 10 | 9 | 16 | 35 | Included 46 adults. | |||||
| Lopez-Sastre et al (131) | Jul 97–Dec 98 | 2003 | Spain | 28 | 62 | 28 | 118 | 3/28 | 5/62 | 12/118 | |||
| Roilides et al (132) | Oct 94–Dec 00, | 2004 | Greece | Mean 1457g | Mean 30 wks | 9 | 38 | 12 | 59 | 1/9 | 15/38 | 17/59 | |
| Giusiano et al (133) | Sept 99–01 | 2004 | Argentina | 27 | 32 | 33 | 92 | ||||||
| Shetty et al (134) | Oct 98–Sep 00 | 2005 | USA | Median 685g | Median 25 wks | 9 | 19 | 7 | 35 | 7/35 | |||
| Benjamin et al 2006 (29) Candidemia | Sept 1998–Dec 31 2001 | 2006 | USA | < 1000 g | 127 | 147 | 33 | 307 | 25/127 | 63/147 | 97/307 | ||
| Benjamin et al 2006 (29) meningitis | Sept 1998–Dec 31 2001 | 2006 | USA | < 1000 g | 5 | 20 | 2 | 27 | 1/5 | 7/20 | 8/27 | ||
| Clerihew 2006 (135) | Feb 03–Feb 04 | 2006 | Great Britain | VLBW infants | 8 | 41 | 9 | 58 | |||||
| Fridkin 2006 (9) | Jan 95–Dec 04 | 2006 | USA | 674 | 1157 | 166 | 1997 | 67/674 | 150/1157 | 260/1997 | |||
| Blyth, 2009 (136) | Aug 01–Jul 04 | 2009 | Australia | 14 | 13 | 6 | 33 | 7/33 | |||||
| Howell 2009 (137) | 1993–2006 | 2009 | Australia | 39 | 74 | 5 | 118 | 19/118 | Study on Oral nystatin | ||||
| Al-Sweih (138) | Jan 95–Dec 06 | 2009 | Kuwait | Mean 1402g | Mean 31wks | 90 | 75 | 17 | 182 | 33/112 | |||
| Motagna et al (139) | Feb 07–Aug 08 | 2010 | Italy | 13 | 7 | 1 | 21 | 5/21 | AURORA Project | ||||
| Kristaf et al (140) | 3 yrs | 2010 | Hungary | 10 | 30 | 1 | 41 | ||||||
| Altuncu et al (141) | 13 yrs | 2010 | Turkey | Median 1735g | Median 33 wks | 12 | 36 | 6 | 54 | ||||
| Rodriguez, et al (25) | Jan 02–Dec 03 | 2010 | Spain | 16 | 7 | 1 | 24 | ||||||
| Peman et al (142) | Jan 09–Feb10 | 2011 | Spain | 24 | 38 | 10 | 72 | ||||||
CP- C. parapsilosis, CA- C. albicans and CT-OT- Others which include C. tropicalis, C. glabrata, C. lusitaniae, C. krusei, C. dublinensis and C. rugosa
Data Synthesis and Analysis
The extracted C. parapsilosis incidence and mortality rates were synthesized and summarized by meta-analyses. Expecting considerable heterogeneity in the included studies, which were mostly observational, we used the random-effects model and the inverse variance method for meta-analysis. Variances and binomial 95% confidence intervals for incidence and mortality rates were calculated using the statistical software STATA, version 11 (StataCorp, College Station, USA). The software Review Manager (RevMan, Version 5.1., Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011) was used for meta-analyses and generation of forest plots. We assessed heterogeneity between studies by visually assessing the forest plots for degree of overlap of confidence intervals and formally estimated statistical heterogeneity by the chi squared statistic (28). Inconsistency across studies was calculated by I2 test (28). In subgroup analyses; we calculated the percentage of C. parapsilosis infections in studies performed before and after the year 2000 to analyze temporal trends and also percentage by geographical regions.
Results
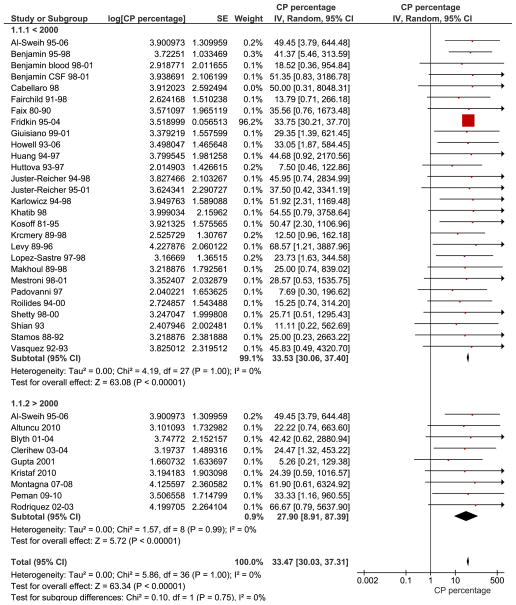
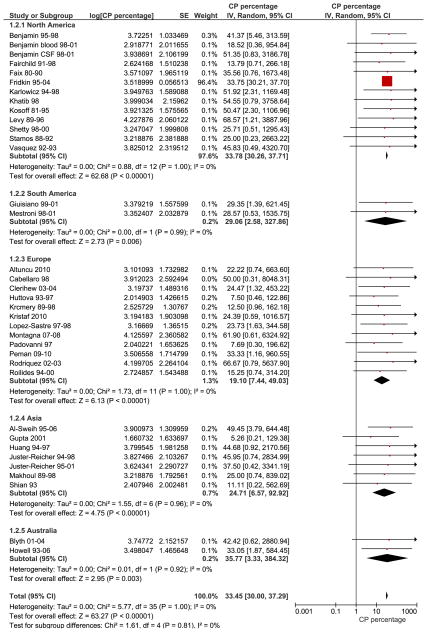
The combined percentage of C. parapsilosis infections of all neonatal Candida infections estimated from 37 studies was 33.47% [95% CI, 30.03, 37.31] (Fig. 1). Subgroup analysis showed that there was no significant difference in the studies performed before the year 2000, 33.53 % [30.06, 37.40] (28 studies) compared to those performed after the year 2000, 27.90 % [8.91, 88.39] (9 studies, wide and overlapping confidence intervals). The largest study in our review was Fridkin et al, which was weighted based on sample size and influenced the outcome the most (9). Sensitivity analyses performed by eliminating the study by Fridkin et al changed the summary estimate insignificantly and with larger confidence intervals, 26.97% [95% CI 15.38, 47.29]. Studies were also sub grouped into 5 different regions to evaluate geographic trends; North America (12 studies), South America (2 studies), Europe (12 studies), Asia (7 studies) and Australia (2 studies) (Fig. 2). C. parapsilosis infection percentages were lowest in Europe 19.10% [95% CI 7.44, 49.03] followed by Asia 24.71% [95% CI 6.57, 92.92], South America 29.06% [95% CI 2.58, 327.86], North America 33.78% [95% CI 30.26, 37.71] and Australia 35.77% [95% CI 3.33, 384.32]. The mortality due to neonatal C. parapsilosis infections was 10.02 [95% CI, 7.66, 13.12] from 10 studies (Candidemia and meningitis data from Benjamin et al (29) were analyzed separately) (Fig. 3). During the same period, the mortality of C. albicans infections from 11 studies was 12.97% [95% CI, 12.65, 13.29] and mortality from all Candida infections was 14.50% [9.54, 22.03] from 23 studies. We did not find significant statistical heterogeneity in the evaluated outcomes among included studies (Chi squared test, p values > 0.10) and I2 statistic for inconsistency not significant for any of the analyses.
Fig. 1. Forest plot depicting percentage of C. parapsilosis infections.
Red squares and black horizontal lines through the squares represent proportion with 95% confidence intervals. First subgroup labeled 1.1.1 represents studies that were predominantly performed before the year 2000 and the second subgroup labeled 1.1.2 represents studies performed after the year 2000. Abbreviations: CP – Candida parapsilosis, SE- standard error, IV- inverse variance, Random-Random effects model for meta-analyses, CI- confidence intervals.
Fig. 2. Forest plot of percentage of C. parapsilosis infections by geographical regions.
Red squares and black horizontal lines through the squares represent proportion with 95% confidence intervals. Studies were sub grouped into 5 different regions; North America (12 studies), South America (2 studies), Europe (12 studies), Asia (7 studies) and Australia (2 studies). Abbreviations: CP – Candida parapsilosis, SE- standard error, IV- inverse variance, Random-Random effects model for meta-analyses, CI- confidence intervals.
Fig. 3. Mortality of C. parapsilosis infections.
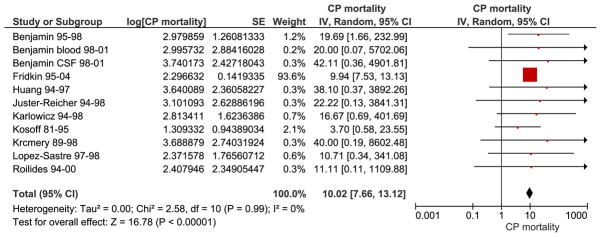
Red squares and black horizontal lines through the squares represent proportion with 95% confidence intervals. The combined mortality of neonatal C. parapsilosis infection was 10.02 [95% CI 7.66, 13.12]. Abbreviations: CP – Candida parapsilosis, SE- standard error, IV- inverse variance, Random- Random effects model for meta-analyses, CI- confidence intervals.
Genetics and Molecular Characteristics of C. parapsilosis
Until recently, before the year 2005, C. parapsilosis isolates were classified into Groups I, II and III (30, 31) but evidence has identified sufficient genetic differences to support the designation of each group as a separate species. Group I isolates remained as C. parapsilosis sensu stricto and Groups II and III were renamed C. orthopsilosis and C. metapsilosis respectively (32). Other species may yet be identified; sequencing of the internal transcriber (ITS) region of ribosomal DNA and the mating type loci of several isolates suggests that there are at least 4 groups represented by C. parapsilosis isolates (33, 34). All C. parapsilosis species are members of the “CTG clade”, which translate the codon “CTG” as serine rather than leucine (35). C. parapsilosis is the most common clinical isolate but 1 to 24% of these isolates may actually be C. orthopsilosis, misidentified as C. parapsilosis (36, 37). In addition, Lodderomyces elongisporus, a more distant relative of the C. parapsilosis group, may be responsible for up to 0.8% of infections previously assigned to C. parapsilosis (38). C. metapsilosis is an environmental organism, rarely isolated from clinical specimens and is less virulent than C. parapsilosis in several models of infection (39, 40).
The genome of C. parapsilosis was sequenced in 2009 along with 5 other Candida species. The diploid chromosomes of C. parapsilosis are highly homogeneous, with a single nucleotide polymorphism (SNP) frequency of about 1/15000, compared to 1/200 in L. elongisporus (41). Individual isolates of C. parapsilosis are also very similar, and are difficult to distinguish except by microsatellite analysis (42, 43). Early annotations of the C. parapsilosis genome were used for large-scale comparative studies (41, 44) and for the design and application of transcriptional microarrays (45, 46). C. parapsilosis shares some features with other pathogenic Candida species, such as an enrichment of cell wall families, including Glycosylphosphatidylinositol (GPI)-anchored adhesins (41). However, there are also substantial differences; for example, unlike C. albicans, the CFEM (common in fungal extracellular membranes) family of cell wall genes in C. parapsilosis is not associated with adhesion or biofilm development (47). Recently, RNA-seq analysis was used to generate a comprehensive and detailed annotation of the C. parapsilosis genome, and to determine the transcriptional response to hypoxic conditions (48). Almost 400 new protein-coding genes were identified, together with many novel transcriptional active regions (nTARs). In addition, comparison with the recently sequenced genome of C. orthopsilosis suggests that the Hyr/Iff family of cell wall proteins is expanded in C. parapsilosis, which may be associated with virulence. There is also substantial expansion of multidrug transporter families (49).
Molecular genetic studies of C. parapsilosis using gene knockouts have been hindered by the fact that the genome is diploid (42, 50, 51) and the lack of a characterized sexual cycle (34). Prior to 2007 an efficient method for targeted gene disruption of C. parapsilosis did not exist. Two groups subsequently adapted a dominant nourseothricin resistance marker developed for C. albicans (52, 53) to knock out lipase genes, and a regulator of biofilm development (54, 55). Although this method is efficient, it is very slow; the resistance marker must first be recycled from the first allele before it can be used to disrupt the second. More rapid methods have been developed in C. albicans either by the use of transposon-directed mutagenesis (56–58), or by using two different auxotrophic markers to target each of the two alleles (59). Screening collections of deletions of transcription factors and protein kinases in C. albicans led to the identification of genes and networks involved in biofilm development, virulence and iron metabolism (60–63). A similar set of deletions is currently being constructed in C. parapsilosis (Holland et al. unpublished data). It is likely that screening these deletions will lead to the identification of species-specific factors in C. parapsilosis that are important for virulence.
Virulence Determinants in C. parapsilosis
Virulence factors identified in C. albicans include adherence, hyphal morphogenesis, biofilm formation and secretion of enzymatic hydrolases including proteases, phospholipases and lipases. However, virulence determinants in C. parapsilosis have not been well characterized. Adherence to epithelial tissues that facilitates colonization of biomaterials and initiation of biofilm formation may be important virulence determinants. Enhanced adherence of C. albicans to neonatal buccal epithelial cells, especially those from premature neonates may increase the risk of oral candidiasis (64, 65). C. parapsilosis adheres to epithelial cells and biomaterials similarly to C. albicans (66–68) and that adherence can be decreased by nystatin (68). Strain variations in adherence have been reported with superficial skin isolates more adherent than systemic isolates (66).
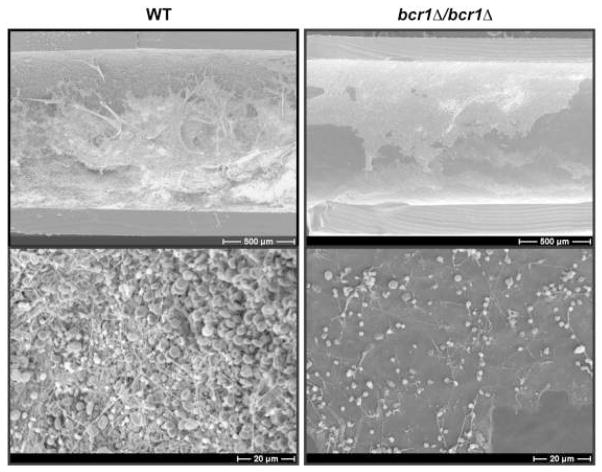
Biofilm formation is an important virulence determinant in C. parapsilosis. Biofilms are sessile microbial communities, adherent to a surface and encased in an extracellular matrix composed of polysaccharides, proteins and extracellular DNA (69). Biofilm developmental stages include adhesion, maturation and dispersal. Main risk factors for C. parapsilosis infection are the presence of indwelling vascular catheters and administration of parenteral nutrition, both of which predispose to the formation of catheter biofilms. In C. albicans, biofilm morphology consists of a compact basal yeast layer and a thicker but less compact hyphal layer (70). C. parapsilosis forms only pseudohyphae (not true hyphae) and its biofilms are thinner and less complex than C. albicans (71). Although C. parapsilosis biofilms are thinner than C. albicans biofilms, antifungal drug resistance to amphotericin and azoles is similar to C. albicans biofilms (72, 73). Hyphal morphogenesis is essential for biofilm formation and virulence in C. albicans (63, 74, 75). Amino acids stimulate morphogenesis from yeast cells to pseudohyphae in C. parapsilosis (76), and this may explain the high incidence of C. parapsilosis infections in catheterized neonates who are on amino acid rich parenteral nutrition solutions. Recent studies of C. parapsilosis biofilms have shown that BCR1, a transcription factor essential for the expression of cell surface antigens, is required for biofilm development both in vitro and in vivo (47, 55) (Fig. 4).
Fig. 4. Electron microscopy of in vivo biofilms.
Biofilm formation on central venous catheters inserted into rats and inoculated with C. parapsilosis (wild type strain CLIB214) and a bcr1 deletion (CDb71) strain. The catheters were removed after 24 hrs and visualized using scanning electron microscopy at two different magnifications. The wild type strain formed a thick biofilm, whereas the bcr1 deletion strain displayed impaired biofilm formation, indicating the essential role of the transcription factor BCR1 in biofilm formation. [Reproduced with permission from Ding C, Vidanes GM, Maguire SL, Guida A, Synnott JM, Andes DR and Geraldine Butler. Conserved and divergent roles of Bcr1 and CFEM proteins in Candida parapsilosis and Candida albicans. PLoS One. 2011;6(12):e28151].
Secreted hydrolytic enzymes such as secreted aspartic proteases (SAP), phospholipases and lipases may cause tissue destruction and initiate pathogenicity (77). However, C. parapsilosis has been shown to have lower SAP activity than C. albicans (78). C. parapsilosis isolates vary in SAP activity, with skin and vulvovaginal isolates having more activity than blood isolates, which may indicate niche-specific adaptation of this organism (79–81). Environmental and epigenetic factors that may regulate the expression of SAPs in different host niches need to be explored. A recent study analyzed the role of C. parapsilosis secreted aspartyl proteinase isoenzyme 1 (SAPP1) in virulence (82). The SAPP1 mutant strain was hypersusceptible to human serum and was attenuated in its capacity to damage host-effector cells. The phagocytosis and killing of mutant cells by human macrophages was significantly enhanced relative to wild type (82). The role of lipases in C. parapsilosis (CpLIP1 and CpLIP2) has also been studied. Lipase mutants form less biofilms and are less virulent in animal models of infection (54). In addition, lipase inhibitors decrease tissue damage in reconstituted human skin epidermal tissues (40). The role of phospholipases in the virulence of C. parapsilosis infections is not clear (79, 83, 84).
Antifungal Susceptibility Patterns of C. parapsilosis and Therapeutic Choices
Amphotericin B remains the mainstay in the therapy of neonatal invasive candidiasis including C. parapsilosis infections. C. parapsilosis resistance in vitro to amphotericin B has been reported but its clinical relevance is not clear (85). Fluconazole prophylaxis in VLBW preterm infants has been shown to be effective in decreasing invasive Candida infections and a composite outcome of invasive candidiasis or mortality (86–88). These results have led to wide adoption of targeted fluconazole prophylaxis strategy in neonatal intensive care units for VLBW infants to prevent invasive candidiasis (86, 87). Controversy remains as to whether widespread use of fluconazole in neonatal units has increased azole resistance among Candida isolates or altered the epidemiology of Candida infections towards non-albicans Candida infections. However, epidemiological studies so far suggest that there is very little change in azole resistance in clinical isolates of Candida infections (89). In a non-human primate neonatal intensive care unit, fluconazole prophylaxis for a period of 4 years was associated with fluconazole-resistant C. parapsilosis infections (90). In a neonatal intensive care unit in Finland, long-term fluconazole prophylaxis has resulted in persistence of a fluconazole-resistant strain of C. parapsilosis causing repeated infections (91). In a study of 409 ELBW infants compared to historical controls, fluconazole prophylaxis significantly decreased invasive Candida infections and mortality due to Candida infections (92). In this study where fluconazole prophylaxis was continued for 4 years, no fluconazole-resistant Candida isolates or change in the epidemiology of Candida infections was observed. In another study, fluconazole prophylaxis targeted to VLBW infants on broad-spectrum antibiotics (n=206) and compared to a historical control (n=178), fluconazole prophylaxis significantly decreased invasive fungal infections and was cost-effective. Most of the infections in the control group (no fluconazole prophylaxis) were caused by C. parapsilosis (93). Some C. parapsilosis isolates (1.5 to 4%) show in vitro resistance to itraconazole, an azole that is rarely used in neonates (85). Approximately 1.9% of C. parapsilosis strains are resistant to voriconazole in vitro but most fluconazole-resistant strains are sensitive to voriconazole (89). The echinocandins, caspofungin, micafungin and anidulafungin, though not the first choice in the treatment of neonatal invasive candidiasis, may be useful in resistant cases. C. parapsilosis isolates show increased echinocandin minimum inhibitory concentrations (MIC) in vitro (85) but the clinical relevance of this is unclear as echinocandins have been effective in vivo (94–96). However, breakthrough infections have occurred in patients on caspofungin therapy. Caspofungin concentrations above the MIC paradoxically promote growth of C. parapsilosis in some instances (97). Increased caspofungin usage has also been associated with increased incidence of C. parapsilosis fungemia (98). These observations raise concern regarding widespread usage of caspofungin in neonates for the fear of selecting resistant C. parapsilosis infections.
Management Strategies for Neonatal C. parapsilosis Infections
A typical patient with invasive C. parapsilosis infection is a preterm VLBW infant with a central line receiving parenteral nutrition and on antibiotics. Management includes removal of the central venous line and systemic amphotericin B therapy. Delayed removal of central line in patients with candidemia increased the duration of blood culture positivity by a median of 3 days irrespective of the Candida species and increased mortality in C. albicans infections (99). In this study, it is also noteworthy that there was a significant difference of mortality between C. albicans and C. parapsilosis infections (24 vs. 4%). In a prospective cohort study of over 4500 infants born at < 1000 g from the National Institute of Child Health and Human Development sponsored Neonatal Research Network, delayed (≥2 days) removal of catheter in infants with positive blood cultures for Candida was associated with increased death or neurodevelopmental impairment in multivariate regression analysis [odds ratio 2.69 (1.25–5.79), p=0.01] (29). Also a trend toward delayed clearance of Candida from the blood was observed in the delayed removal group; 7.3 vs. 5 days, p=0.11. Persistence of candidemia for 5 days or more is associated with an increased risk of ophthalmologic, renal or cardiac dissemination compared to infants with lower duration of candidemia (100). Hence prompt removal of the infected central venous catheter is recommended after diagnosis of candidemia.
Systemic amphotericin B therapy is continued via a peripheral intravenous line until blood cultures are clear of C. parapsilosis for at least 2 cultures, after which replacement of the central venous line can be considered. Also, in any patient with an invasive fungal infection, additional foci of infection should be explored by cultures of urine and CSF, echocardiogram, fundoscopy and sonograms of the kidneys and the liver. Liposomal amphotericin may be an option in neonates with renal or hepatic dysfunction and in vitro studies demonstrate higher efficacy of liposomal amphotericin against biofilms of Candida than amphotericin B (101–103). However, a recently published large retrospective cohort study with 730 neonates with candidiasis has reported higher mortality and higher therapeutic failure rates in neonates treated with amphotericin lipid products (that includes liposomal amphotericin) compared to conventional amphotericin or fluconazole (104). Nearly a fourth of invasive Candida infections are associated with a concurrent bacterial infection (often Coagulase negative Staphylococci and Enterococci) that need appropriate antibiotic therapy (100, 105, 106).
Persistence of C. parapsilosis fungemia in spite of removal of central venous line should prompt evaluation of antifungal susceptibility of the isolate and consideration of echinocandins (caspofungin or micafungin) as ‘add on’ or replacement therapy. Combination of amphotericin B with echinocandins is effective in vitro but there are very few studies that have tested them in vivo (107). Duration of antifungal therapy is 2 to 3 weeks after the last positive blood culture in candidemia and 1–2 weeks for isolated urinary tract infection (108). However, Candida in the urine may be the first manifestation of candidemia and hence systemic evaluation for candidemia is necessary. Longer duration of therapy of 4 weeks is considered in the presence of meningitis, 6–12 weeks for endopthalmitis and at least 6 weeks in endocarditis with or without surgical therapy. Empirical antifungal therapy has been advocated in neonates at high risk of nosocomial infections such as those on cephalosporins or other clinical and laboratory parameters but needs careful consideration (109).
Prevention of C. parapsilosis Infections
Prevention should target the horizontal transmission of C. parapsilosis in the neonatal unit. Monitoring and surveillance for C. parapsilosis infections, awareness and compliance with hand hygiene, bundled strategies for prevention of central venous catheter infections and antifungal prophylaxis are important strategies (110). The benefit of isolation measures such as cohorting or single room isolation of neonates who are colonized or infected with Candida is not clear (111). It is also paramount to create awareness, institute educational policies for health care staff and provide feedback as a part of a quality improvement initiative.
General preventive strategies include initiation of early human milk feeding to decrease dependence on central venous lines and parenteral nutrition. Judicious use of antibiotics, avoiding broad-spectrum antibiotics such as cephalosporins, steroids, H2 blockers and proton pump inhibitors is recommended. Antifungal prophylaxis strategies may be useful in decreasing colonization and subsequent invasive fungal infections. A meta-analyses of 638 infants in 7 trials found that prophylactic fluconazole significantly decreased invasive fungal infection [RR 0.23 (95% confidence interval 0.11, 0.46)] but not mortality prior to hospital discharge [RR: 0.61 (95% confidence interval 0.37, 1.03)] (88). However, the baseline fungal infection rate was high in the placebo arm in the included trials. Targeted fluconazole prophylaxis in VLBW or ELBW infants with risk factors is an alternative strategy that may be effective. Follow-up of ELBW infants at 8–10 years, who received fluconazole prophylaxis in the neonatal period revealed no adverse effects on neurodevelopment or quality of life (112). Another meta-analysis of 1625 infants in 3 trials found that oral or topical non-absorbed antifungal prophylaxis (nystatin or miconazole) significantly decreased invasive fungal infections [RR 0.19 (95% confidence interval (CI) 0.14, 0.27)] but not mortality [RR 0.88 (95% CI 0.72, 1.06)] (113). The choice of nystatin vs. fluconazole prophylaxis in preventing invasive Candida infections including their relative efficacies and safety has been debated (114).
Conclusions
C. parapsilosis infections are a significant problem in the premature neonate and contribute significantly to neonatal mortality and morbidity. C. parapsilosis infections are responsible for a third of neonatal Candida infections and have a mortality rate of approximately 10%. The reasons for predilection of C. parapsilosis infection in neonates are not clear but adherence to skin and biomaterials leading to biofilm formation may be important determinants. Advances in microbial genetics and availability of tools for genetic manipulation will help us understand the virulence and other organism characteristics responsible for neonatal pathogenicity of C. parapsilosis. Amphotericin B remains the antifungal drug of choice and combination therapy with caspofungin or other echinocandins may be considered in resistant cases. Early enteral feeding with human milk and early removal of central venous lines, avoidance of steroids, H2 blockers, judicious use of antibiotics and antifungal prophylaxis may decrease C. parapsilosis infections.
Table 1.
| Years of publication | Number of publications |
|---|---|
| 1990–95 | 22 |
| 1996–2000 | 32 |
| 2001–05 | 68 |
| 2006–2010 | 79 |
Increasing awareness and reports of C. parapsilosis infections in neonates observed on review of literature. We searched for publications using the search terms ‘Candida parapsilosis’ and ‘neonat*’ and plotted in epochs of 5 years.
Acknowledgments
Funding: GB is supported by Science foundation, Ireland. AG is supported by OTKA NF84006, NN100374 and by EMBO Installation Grant 1813. Work in Dr. Bliss’ laboratory was supported by grants from the National Center for Research Resources (5P20RR018728-10) and the National Institute of General Medical Sciences (8P20GM103537-10) from the National Institutes of Health.
Footnotes
Financial disclosure and conflict of interest: None of the authors have financial or other conflicts of interest to disclose
References
- 1.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 3.Almirante B, Rodriguez D, Cuenca-Estrella M, et al. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2006;44:1681–1685. doi: 10.1128/JCM.44.5.1681-1685.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brito LR, Guimaraes T, Nucci M, et al. Clinical and microbiological aspects of candidemia due to Candida parapsilosis in Brazilian tertiary care hospitals. Med Mycol. 2006;44:261–266. doi: 10.1080/13693780500421476. [DOI] [PubMed] [Google Scholar]
- 5.Krcmery V, Barnes AJ. Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J Hosp Infect. 2002;50:243–260. doi: 10.1053/jhin.2001.1151. [DOI] [PubMed] [Google Scholar]
- 6.Messer SA, Jones RN, Fritsche TR. International surveillance of Candida spp. and Aspergillus spp.: report from the SENTRY Antimicrobial Surveillance Program (2003) J Clin Microbiol. 2006;44:1782–1787. doi: 10.1128/JCM.44.5.1782-1787.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neu N, Malik M, Lunding A, et al. Epidemiology of candidemia at a Children’s hospital, 2002 to 2006. Pediatr Infect Dis J. 2009;28:806–809. doi: 10.1097/INF.0b013e3181a0d78d. [DOI] [PubMed] [Google Scholar]
- 8.Medrano DJ, Brilhante RS, de Cordeiro RA, et al. Candidemia in a Brazilian hospital: the importance of Candida parapsilosis. Rev Inst Med Trop Sao Paulo. 2006;48:17–20. doi: 10.1590/s0036-46652006000100004. [DOI] [PubMed] [Google Scholar]
- 9.Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995–2004. Pediatrics. 2006;117:1680–1687. doi: 10.1542/peds.2005-1996. [DOI] [PubMed] [Google Scholar]
- 10.Kossoff EH, Buescher ES, Karlowicz MG. Candidemia in a neonatal intensive care unit: trends during fifteen years and clinical features of 111 cases. Pediatr Infect Dis J. 1998;17:504–508. doi: 10.1097/00006454-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Trofa D, Gacser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21:606–625. doi: 10.1128/CMR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali GY, Algohary EH, Rashed KA, Almoghanum M, Khalifa AA. Prevalence of Candida colonization in preterm newborns and VLBW in neonatal intensive care unit: role of maternal colonization as a risk factor in transmission of disease. J Matern Fetal Neonatal Med. 2012;25:789–795. doi: 10.3109/14767058.2011.622005. [DOI] [PubMed] [Google Scholar]
- 13.Bliss JM, Basavegowda KP, Watson WJ, Sheikh AU, Ryan RM. Vertical and horizontal transmission of Candida albicans in very low birth weight infants using DNA fingerprinting techniques. Pediatr Infect Dis J. 2008;27:231–235. doi: 10.1097/INF.0b013e31815bb69d. [DOI] [PubMed] [Google Scholar]
- 14.Mahieu LM, Van Gasse N, Wildemeersch D, Jansens H, Ieven M. Number of sites of perinatal Candida colonization and neutropenia are associated with nosocomial candidemia in the neonatal intensive care unit patient. Pediatr Crit Care Med. 2010;11:240–245. doi: 10.1097/PCC.0b013e3181b808fb. [DOI] [PubMed] [Google Scholar]
- 15.Manzoni P, Farina D, Galletto P, et al. Type and number of sites colonized by fungi and risk of progression to invasive fungal infection in preterm neonates in neonatal intensive care unit. J Perinat Med. 2007;35:220–226. doi: 10.1515/JPM.2007.055. [DOI] [PubMed] [Google Scholar]
- 16.Bendel CM. Colonization and epithelial adhesion in the pathogenesis of neonatal candidiasis. Semin Perinatol. 2003;27:357–364. doi: 10.1016/s0146-0005(03)00059-4. [DOI] [PubMed] [Google Scholar]
- 17.Clerihew L, Lamagni TL, Brocklehurst P, McGuire W. Candida parapsilosis infection in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:F127–129. doi: 10.1136/fnn.2006.097758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saiman L, Ludington E, Dawson JD, et al. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr Infect Dis J. 2001;20:1119–1124. doi: 10.1097/00006454-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman DA, Gurka MJ, Hazen KC, et al. Patterns of fungal colonization in preterm infants weighing less than 1000 grams at birth. Pediatr Infect Dis J. 2006;25:733–737. doi: 10.1097/01.inf.0000226978.96218.e6. [DOI] [PubMed] [Google Scholar]
- 20.el-Mohandes AE, Johnson-Robbins L, Keiser JF, Simmens SJ, Aure MV. Incidence of Candida parapsilosis colonization in an intensive care nursery population and its association with invasive fungal disease. Pediatr Infect Dis J. 1994;13:520–524. doi: 10.1097/00006454-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Lupetti A, Tavanti A, Davini P, et al. Horizontal transmission of Candida parapsilosis candidemia in a neonatal intensive care unit. J Clin Microbiol. 2002;40:2363–2369. doi: 10.1128/JCM.40.7.2363-2369.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonassoli LA, Bertoli M, Svidzinski TI. High frequency of Candida parapsilosis on the hands of healthy hosts. J Hosp Infect. 2005;59:159–162. doi: 10.1016/j.jhin.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 23.van Asbeck EC, Huang YC, Markham AN, Clemons KV, Stevens DA. Candida parapsilosis fungemia in neonates: genotyping results suggest healthcare workers hands as source, and review of published studies. Mycopathologia. 2007;164:287–293. doi: 10.1007/s11046-007-9054-3. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Castro R, Arroyo-Escalante S, Carrillo-Casas EM, et al. Outbreak of Candida parapsilosis in a neonatal intensive care unit: a health care workers source. Eur J Pediatr. 2010;169:783–787. doi: 10.1007/s00431-009-1109-7. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez D, Almirante B, Cuenca-Estrella M, et al. Predictors of candidaemia caused by non-albicans Candida species: results of a population-based surveillance in Barcelona, Spain. Clin Microbiol Infect. 2010;16:1676–1682. doi: 10.1111/j.1469-0691.2010.03208.x. [DOI] [PubMed] [Google Scholar]
- 26.Miranda LN, van der Heijden IM, Costa SF, et al. Candida colonisation as a source for candidaemia. J Hosp Infect. 2009;72:9–16. doi: 10.1016/j.jhin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Green S, editors. Handbook for Systematic Reviews of Interventions, Version 5.1.0. The Cochrane Collaboration, 2011. 2011 Available from www.cochrane-handbook.org.
- 29.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 30.Lin D, Wu LC, Rinaldi MG, Lehmann PF. Three distinct genotypes within Candida parapsilosis from clinical sources. J Clin Microbiol. 1995;33:1815–1821. doi: 10.1128/jcm.33.7.1815-1821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lott TJ, Kuykendall RJ, Welbel SF, Pramanik A, Lasker BA. Genomic heterogeneity in the yeast Candida parapsilosis. Curr Genet. 1993;23:463–467. doi: 10.1007/BF00312635. [DOI] [PubMed] [Google Scholar]
- 32.Tavanti A, Davidson AD, Gow NA, Maiden MC, Odds FC. Candida orthopsilosis and Candida metapsilosis spp.nov. to replace Candida parapsilosis groups II and III. J Clin Microbiol. 2005;43:284–292. doi: 10.1128/JCM.43.1.284-292.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iida S, Imai T, Oguri T, et al. Genetic diversity of the internal transcribed spacers (ITS) and 5.8S rRNA genes among the clinical isolates of Candida parapsilosis in Brazil and Japan. Nihon Ishinkin Gakkai Zasshi. 2005;46:133–137. doi: 10.3314/jjmm.46.133. [DOI] [PubMed] [Google Scholar]
- 34.Sai S, Holland LM, McGee CF, Lynch DB, Butler G. Evolution of mating within the Candida parapsilosis species group. Eukaryot Cell. 2011;10:578–587. doi: 10.1128/EC.00276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos MA, Tuite MF. The CUG codon is decoded in vivo as serine and not leucine in Candida albicans. Nucleic Acids Res. 1995;23:1481–1486. doi: 10.1093/nar/23.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Lopez A, Alastruey-Izquierdo A, Rodriguez D, et al. Prevalence and susceptibility profile of Candida metapsilosis and Candida orthopsilosis: results from population-based surveillance of candidemia in Spain. Antimicrobial agents and chemotherapy. 2008;52:1506–1509. doi: 10.1128/AAC.01595-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tay ST, Na SL, Chong J. Molecular differentiation and antifungal susceptibilities of Candida parapsilosis isolated from patients with bloodstream infections. J Med Microbiol. 2009;58:185–191. doi: 10.1099/jmm.0.004242-0. [DOI] [PubMed] [Google Scholar]
- 38.Lockhart SR, Messer SA, Pfaller MA, Diekema DJ. Lodderomyces elongisporus masquerading as Candida parapsilosis as a cause of bloodstream infections. Journal of clinical microbiology. 2008;46:374–376. doi: 10.1128/JCM.01790-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tavanti A, Hensgens LA, Mogavero S, et al. Genotypic and phenotypic properties of Candida parapsilosis sensu strictu strains isolated from different geographic regions and body sites. BMC Microbiol. 2010;10:203. doi: 10.1186/1471-2180-10-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gacser A, Schafer W, Nosanchuk JS, Salomon S, Nosanchuk JD. Virulence of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis in reconstituted human tissue models. Fungal Genet Biol. 2007;44:1336–1341. doi: 10.1016/j.fgb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Butler G, Rasmussen MD, Lin MF, et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–662. doi: 10.1038/nature08064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lasker BA, Butler G, Lott TJ. Molecular genotyping of Candida parapsilosis group I clinical isolates by analysis of polymorphic microsatellite markers. Journal of clinical microbiology. 2006;44:750–759. doi: 10.1128/JCM.44.3.750-759.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabino R, Sampaio P, Rosado L, et al. New polymorphic microsatellite markers able to distinguish among Candida parapsilosis sensu stricto isolates. Journal of clinical microbiology. 2010;48:1677–1682. doi: 10.1128/JCM.02151-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fitzpatrick DA, O’Gaora P, Byrne KP, Butler G. Analysis of gene evolution and metabolic pathways using the Candida Gene Order Browser. BMC Genomics. 2010;11:290. doi: 10.1186/1471-2164-11-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossignol T, Logue ME, Reynolds K, et al. Transcriptional response of Candida parapsilosis following exposure to farnesol. Antimicrob Agents Chemother. 2007;51:2304–2312. doi: 10.1128/AAC.01438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossignol T, Ding C, Guida A, et al. Correlation between biofilm formation and the hypoxic response in Candida parapsilosis. Eukaryotic cell. 2009;8:550–559. doi: 10.1128/EC.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding C, Vidanes GM, Maguire SL, et al. Conserved and divergent roles of Bcr1 and CFEM proteins in Candida parapsilosis and Candida albicans. PLoS One. 2011;6:e28151. doi: 10.1371/journal.pone.0028151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guida A, Lindstadt C, Maguire SL, et al. Using RNA-seq to determine the transcriptional landscape and the hypoxic response of the pathogenic yeast Candida parapsilosis. BMC Genomics. 2011;12:628. doi: 10.1186/1471-2164-12-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riccombeni A, Vidanes G, Proux-Wera E, Wolfe KH, Butler G. Sequence and analysis of the genome of the pathogenic yeast Candida orthopsilosis. PLoS One. 2012;7:e35750. doi: 10.1371/journal.pone.0035750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doi M, Homma M, Chindamporn A, Tanaka K. Estimation of chromosome number and size by pulsed-field gel electrophoresis (PFGE) in medically important Candida species. J Gen Microbiol. 1992;138:2243–2251. doi: 10.1099/00221287-138-10-2243. [DOI] [PubMed] [Google Scholar]
- 51.Fundyga RE, Kuykendall RJ, Lee-Yang W, Lott TJ. Evidence for aneuploidy and recombination in the human commensal yeast Candida parapsilosis. Infect Genet Evol. 2004;4:37–43. doi: 10.1016/j.meegid.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Reuss O, Vik A, Kolter R, Morschhauser J. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene. 2004;341:119–127. doi: 10.1016/j.gene.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 53.Wirsching S, Michel S, Morschhauser J. Targeted gene disruption in Candida albicans wild-type strains: the role of the MDR1 gene in fluconazole resistance of clinical Candida albicans isolates. Mol Microbiol. 2000;36:856–865. doi: 10.1046/j.1365-2958.2000.01899.x. [DOI] [PubMed] [Google Scholar]
- 54.Gacser A, Trofa D, Schafer W, Nosanchuk JD. Targeted gene deletion in Candida parapsilosis demonstrates the role of secreted lipase in virulence. J Clin Invest. 2007;117:3049–3058. doi: 10.1172/JCI32294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding C, Butler G. Development of a gene knockout system in Candida parapsilosis reveals a conserved role for BCR1 in biofilm formation. Eukaryot Cell. 2007;6:1310–1319. doi: 10.1128/EC.00136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis DA, Bruno VM, Loza L, Filler SG, Mitchell AP. Candida albicans Mds3p, a conserved regulator of pH responses and virulence identified through insertional mutagenesis. Genetics. 2002;162:1573–1581. doi: 10.1093/genetics/162.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uhl MA, Biery M, Craig N, Johnson AD. Haploinsufficiency-based large-scale forward genetic analysis of filamentous growth in the diploid human fungal pathogen C.albicans. Embo J. 2003;22:2668–2678. doi: 10.1093/emboj/cdg256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh J, Fung E, Schlecht U, et al. Gene annotation and drug target discovery in Candida albicans with a tagged transposon mutant collection. PLoS Pathog. 2010;6:e1001140. doi: 10.1371/journal.ppat.1001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noble SM, Johnson AD. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryotic cell. 2005;4:298–309. doi: 10.1128/EC.4.2.298-309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen C, Pande K, French SD, Tuch BB, Noble SM. An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe. 2011;10:118–135. doi: 10.1016/j.chom.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noble SM, French S, Kohn LA, Chen V, Johnson AD. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet. 2010;42:590–598. doi: 10.1038/ng.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blankenship JR, Fanning S, Hamaker JJ, Mitchell AP. An extensive circuitry for cell wall regulation in Candida albicans. PLoS Pathog. 2010;6:e1000752. doi: 10.1371/journal.ppat.1000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nobile CJ, Fox EP, Nett JE, et al. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148:126–138. doi: 10.1016/j.cell.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cox F. Candida albicans adherence in newborn infants. J Med Vet Mycol. 1986;24:121–125. [PubMed] [Google Scholar]
- 65.Cox F. Adherence of Candida albicans to buccal epithelial cells in children and adults. J Lab Clin Med. 1983;102:960–972. [PubMed] [Google Scholar]
- 66.Panagoda GJ, Ellepola AN, Samaranayake LP. Adhesion of Candida parapsilosis to epithelial and acrylic surfaces correlates with cell surface hydrophobicity. Mycoses. 2001;44:29–35. doi: 10.1046/j.1439-0507.2001.00611.x. [DOI] [PubMed] [Google Scholar]
- 67.Kojic EM, Darouiche RO. Comparison of adherence of Candida albicans and Candida parapsilosis to silicone catheters in vitro and in vivo. Clin Microbiol Infect. 2003;9:684–690. doi: 10.1046/j.1469-0691.2003.00724.x. [DOI] [PubMed] [Google Scholar]
- 68.Ellepola AN, Panagoda GJ, Samaranayake LP. Adhesion of oral Candida species to human buccal epithelial cells following brief exposure to nystatin. Oral Microbiol Immunol. 1999;14:358–363. doi: 10.1034/j.1399-302x.1999.140605.x. [DOI] [PubMed] [Google Scholar]
- 69.Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. Candida biofilms: an update. Eukaryot Cell. 2005;4:633–638. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baillie GS, Douglas LJ. Role of dimorphism in the development of Candida albicans biofilms. J Med Microbiol. 1999;48:671–679. doi: 10.1099/00222615-48-7-671. [DOI] [PubMed] [Google Scholar]
- 71.Kuhn DM, Chandra J, Mukherjee PK, Ghannoum MA. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun. 2002;70:878–888. doi: 10.1128/iai.70.2.878-888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katragkou A, Chatzimoschou A, Simitsopoulou M, et al. Differential activities of newer antifungal agents against Candida albicans and Candida parapsilosis biofilms. Antimicrob Agents Chemother. 2008;52:357–360. doi: 10.1128/AAC.00856-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruzicka F, Hola V, Votava M, Tejkalova R. Importance of biofilm in Candida parapsilosis and evaluation of its susceptibility to antifungal agents by colorimetric method. Folia Microbiol (Praha) 2007;52:209–214. doi: 10.1007/BF02931300. [DOI] [PubMed] [Google Scholar]
- 74.Paramonova E, Krom BP, van der Mei HC, Busscher HJ, Sharma PK. Hyphal content determines the compression strength of Candida albicans biofilms. Microbiology. 2009;155:1997–2003. doi: 10.1099/mic.0.021568-0. [DOI] [PubMed] [Google Scholar]
- 75.Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim SK, El Bissati K, Ben Mamoun C. Amino acids mediate colony and cell differentiation in the fungal pathogen Candida parapsilosis. Microbiology. 2006;152:2885–2894. doi: 10.1099/mic.0.29180-0. [DOI] [PubMed] [Google Scholar]
- 77.Schaller M, Borelli C, Korting HC, Hube B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48:365–377. doi: 10.1111/j.1439-0507.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- 78.Ruchel R, Boning B, Borg M. Characterization of a secretory proteinase of Candida parapsilosis and evidence for the absence of the enzyme during infection in vitro. Infect Immun. 1986;53:411–419. doi: 10.1128/iai.53.2.411-419.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dagdeviren M, Cerikcioglu N, Karavus M. Acid proteinase, phospholipase and adherence properties of Candida parapsilosis strains isolated from clinical specimens of hospitalised patients. Mycoses. 2005;48:321–326. doi: 10.1111/j.1439-0507.2005.01145.x. [DOI] [PubMed] [Google Scholar]
- 80.De Bernardis F, Arancia S, Morelli L, et al. Evidence that members of the secretory aspartyl proteinase gene family, in particular SAP2, are virulence factors for Candida vaginitis. J Infect Dis. 1999;179:201–208. doi: 10.1086/314546. [DOI] [PubMed] [Google Scholar]
- 81.De Bernardis F, Sullivan PA, Cassone A. Aspartyl proteinases of Candida albicans and their role in pathogenicity. Med Mycol. 2001;39:303–313. doi: 10.1080/mmy.39.4.303.313. [DOI] [PubMed] [Google Scholar]
- 82.Horvath P, Nosanchuk JD, Hamari Z, Vagvolgyi C, Gacser A. The identification of gene duplication and the role of secreted aspartyl proteinase 1 in Candida parapsilosis virulence. J Infect Dis. 2012;205:923–933. doi: 10.1093/infdis/jir873. [DOI] [PubMed] [Google Scholar]
- 83.Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev. 2000;13:122–143. doi: 10.1128/cmr.13.1.122-143.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kantarcioglu AS, Yucel A. Phospholipase and protease activities in clinical Candida isolates with reference to the sources of strains. Mycoses. 2002;45:160–165. doi: 10.1046/j.1439-0507.2002.00727.x. [DOI] [PubMed] [Google Scholar]
- 85.Ostrosky-Zeichner L, Rex JH, Pappas PG, et al. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob Agents Chemother. 2003;47:3149–3154. doi: 10.1128/AAC.47.10.3149-3154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaufman D, Boyle R, Hazen KC, et al. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345:1660–1666. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 87.Kaufman D. Fluconazole prophylaxis decreases the combined outcome of invasive Candida infections or mortality in preterm infants. Pediatrics. 2008;122:1158–1159. doi: 10.1542/peds.2008-1837. [DOI] [PubMed] [Google Scholar]
- 88.Clerihew L, Austin N, McGuire W. Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants. Cochrane Database Syst Rev. 2007:CD003850. doi: 10.1002/14651858.CD003850.pub3. [DOI] [PubMed] [Google Scholar]
- 89.Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol. 2010;48:1366–1377. doi: 10.1128/JCM.02117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoder BA, Sutton DA, Winter V, Coalson JJ. Resistant Candida parapsilosis associated with long term fluconazole prophylaxis in an animal model. Pediatr Infect Dis J. 2004;23:687–688. doi: 10.1097/01.inf.0000128777.22022.47. [DOI] [PubMed] [Google Scholar]
- 91.Sarvikivi E, Lyytikainen O, Soll DR, et al. Emergence of fluconazole resistance in a Candida parapsilosis strain that caused infections in a neonatal intensive care unit. J Clin Microbiol. 2005;43:2729–2735. doi: 10.1128/JCM.43.6.2729-2735.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Healy CM, Campbell JR, Zaccaria E, Baker CJ. Fluconazole prophylaxis in extremely low birth weight neonates reduces invasive candidiasis mortality rates without emergence of fluconazole-resistant Candida species. Pediatrics. 2008;121:703–710. doi: 10.1542/peds.2007-1130. [DOI] [PubMed] [Google Scholar]
- 93.Uko S, Soghier LM, Vega M, et al. Targeted short-term fluconazole prophylaxis among very low birth weight and extremely low birth weight infants. Pediatrics. 2006;117:1243–1252. doi: 10.1542/peds.2005-1969. [DOI] [PubMed] [Google Scholar]
- 94.Odio CM, Araya R, Pinto LE, et al. Caspofungin therapy of neonates with invasive candidiasis. Pediatr Infect Dis J. 2004;23:1093–1097. [PubMed] [Google Scholar]
- 95.Yalaz M, Akisu M, Hilmioglu S, et al. Successful caspofungin treatment of multidrug resistant Candida parapsilosis septicaemia in an extremely low birth weight neonate. Mycoses. 2006;49:242–245. doi: 10.1111/j.1439-0507.2006.01220.x. [DOI] [PubMed] [Google Scholar]
- 96.Deshpande K. Candida parapsilosis fungaemia treated unsuccessfully with amphotericin B and fluconazole but eliminated with caspofungin: a case report. Crit Care Resusc. 2003;5:20–23. [PubMed] [Google Scholar]
- 97.Chamilos G, Lewis RE, Albert N, Kontoyiannis DP. Paradoxical effect of Echinocandins across Candida species in vitro: evidence for echinocandin-specific and candida species-related differences. Antimicrob Agents Chemother. 2007;51:2257–2259. doi: 10.1128/AAC.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Forrest GN, Weekes E, Johnson JK. Increasing incidence of Candida parapsilosis candidemia with caspofungin usage. J Infect. 2008;56:126–129. doi: 10.1016/j.jinf.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 99.Karlowicz MG, Hashimoto LN, Kelly RE, Jr, Buescher ES. Should central venous catheters be removed as soon as candidemia is detected in neonates? Pediatrics. 2000;106:E63. doi: 10.1542/peds.106.5.e63. [DOI] [PubMed] [Google Scholar]
- 100.Noyola DE, Fernandez M, Moylett EH, Baker CJ. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin Infect Dis. 2001;32:1018–1023. doi: 10.1086/319601. [DOI] [PubMed] [Google Scholar]
- 101.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002;46:1773–1780. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Juster-Reicher A, Flidel-Rimon O, Amitay M, et al. High-dose liposomal amphotericin B in the therapy of systemic candidiasis in neonates. Eur J Clin Microbiol Infect Dis. 2003;22:603–607. doi: 10.1007/s10096-003-0993-4. [DOI] [PubMed] [Google Scholar]
- 103.Juster-Reicher A, Leibovitz E, Linder N, et al. Liposomal amphotericin B (AmBisome) in the treatment of neonatal candidiasis in very low birth weight infants. Infection. 2000;28:223–226. doi: 10.1007/s150100070040. [DOI] [PubMed] [Google Scholar]
- 104.Ascher SB, Smith PB, Watt K, et al. Antifungal therapy and outcomes in infants with invasive Candida infections. Pediatr Infect Dis J. 2012;31:439–443. doi: 10.1097/INF.0b013e3182467a72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karlowicz MG, Giannone PJ, Pestian J, Morrow AL, Shults J. Does candidemia predict threshold retinopathy of prematurity in extremely low birth weight (</=1000 g) neonates? Pediatrics. 2000;105:1036–1040. doi: 10.1542/peds.105.5.1036. [DOI] [PubMed] [Google Scholar]
- 106.Fairchild KD, Tomkoria S, Sharp EC, Mena FV. Neonatal Candida glabrata sepsis: clinical and laboratory features compared with other Candida species. Pediatr Infect Dis J. 2002;21:39–43. doi: 10.1097/00006454-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 107.Mukherjee PK, Sheehan DJ, Hitchcock CA, Ghannoum MA. Combination treatment of invasive fungal infections. Clin Microbiol Rev. 2005;18:163–194. doi: 10.1128/CMR.18.1.163-194.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161–189. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- 109.Benjamin DK, Jr, Ross K, McKinney RE, Jr, et al. When to suspect fungal infection in neonates: A clinical comparison of Candida albicans and Candida parapsilosis fungemia with coagulase-negative staphylococcal bacteremia. Pediatrics. 2000;106:712–718. doi: 10.1542/peds.106.4.712. [DOI] [PubMed] [Google Scholar]
- 110.Adams-Chapman I, Stoll BJ. Prevention of nosocomial infections in the neonatal intensive care unit. Curr Opin Pediatr. 2002;14:157–164. doi: 10.1097/00008480-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 111.Mohan P, Eddama O, Weisman LE. Patient isolation measures for infants with candida colonization or infection for preventing or reducing transmission of candida in neonatal units. Cochrane Database Syst Rev. 2007:CD006068. doi: 10.1002/14651858.CD006068.pub2. [DOI] [PubMed] [Google Scholar]
- 112.Kaufman DA, Cuff AL, Wamstad JB, et al. Fluconazole prophylaxis in extremely low birth weight infants and neurodevelopmental outcomes and quality of life at 8 to 10 years of age. J Pediatr. 2011;158:759–765. e751. doi: 10.1016/j.jpeds.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 113.Austin N, Darlow BA, McGuire W. Prophylactic oral/topical non-absorbed antifungal agents to prevent invasive fungal infection in very low birth weight infants. Cochrane Database Syst Rev. 2009:CD003478. doi: 10.1002/14651858.CD003478.pub3. [DOI] [PubMed] [Google Scholar]
- 114.Kaufman DA, Manzoni P. Strategies to prevent invasive candidal infection in extremely preterm infants. Clin Perinatol. 2010;37:611–628. doi: 10.1016/j.clp.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 115.Faix RG. Invasive neonatal candidiasis: comparison of albicans and parapsilosis infection. Pediatr Infect Dis J. 1992;11:88–93. [PubMed] [Google Scholar]
- 116.Shian WJ, Chi CS, Wang TM, Chen CH. Candidemia in the neonatal intensive care unit. Zhonghua Minguo xiao er ke yi xue hui za zhi [Journal] Zhonghua Minguo xiao er ke yi xue hui. 1993;34:349–355. [PubMed] [Google Scholar]
- 117.Saxen H, Virtanen M, Carlson P, et al. Neonatal Candida parapsilosis outbreak with a high case fatality rate. Pediatr Infect Dis J. 1995;14:776–781. doi: 10.1097/00006454-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 118.Stamos JK, Rowley AH. Candidemia in a pediatric population. Clin Infect Dis. 1995;20:571–575. doi: 10.1093/clinids/20.3.571. [DOI] [PubMed] [Google Scholar]
- 119.Padovani EM, Michielutti F, Dall’Agnola A, Dal Moro A, Khoory BJ. Sepsis caused by Candida in the neonatal period. La Pediatria medica e chirurgica : Medical and surgical pediatrics. 1997;19:83–88. [PubMed] [Google Scholar]
- 120.Vazquez JA, Boikov D, Boikov SG, Dajani AS. Use of electrophoretic karyotyping in the evaluation of Candida infections in a neonatal intensive-care unit. Infect Control Hosp Epidemiol. 1997;18:32–37. doi: 10.1086/647498. [DOI] [PubMed] [Google Scholar]
- 121.Huttova M, Hartmanova I, Kralinsky K, et al. Candida fungemia in neonates treated with fluconazole: report of forty cases, including eight with meningitis. Pediatr Infect Dis J. 1998;17:1012–1015. doi: 10.1097/00006454-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 122.Jaraba Caballero S, Jaraba Caballero MP, Fernandez Gutierrez F, et al. Prospective study of Candida-related sepsis in the neonate. Anales espanoles de pediatria. 1998;48:639–643. [PubMed] [Google Scholar]
- 123.Levy I, Rubin LG, Vasishtha S, Tucci V, Sood SK. Emergence of Candida parapsilosis as the predominant species causing candidemia in children. Clin Infect Dis. 1998;26:1086–1088. doi: 10.1086/520277. [DOI] [PubMed] [Google Scholar]
- 124.Khatib R, Thirumoorthi MC, Riederer KM, et al. Clustering of Candida infections in the neonatal intensive care unit: concurrent emergence of multiple strains simulating intermittent outbreaks. Pediatr Infect Dis J. 1998;17:130–134. doi: 10.1097/00006454-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 125.Huang YC, Lin TY, Leu HS, et al. Outbreak of Candida parapsilosis fungemia in neonatal intensive care units: clinical implications and genotyping analysis. Infection. 1999;27:97–102. doi: 10.1007/BF02560505. [DOI] [PubMed] [Google Scholar]
- 126.Huang YC, Lin TY, Lien RI, et al. Candidaemia in special care nurseries: comparison of albicans and parapsilosis infection. J Infect. 2000;40:171–175. doi: 10.1053/jinf.2000.0638. [DOI] [PubMed] [Google Scholar]
- 127.Krcmery V, Fric M, Pisarcikova M, et al. Fungemia in neonates: report of 80 cases from seven University hospitals. Pediatrics. 2000;105:913–914. doi: 10.1542/peds.105.4.913. [DOI] [PubMed] [Google Scholar]
- 128.Gupta N, Mittal N, Sood P, et al. Candidemia in neonatal intensive care unit. Indian J Pathol Microbiol. 2001;44:45–48. [PubMed] [Google Scholar]
- 129.Makhoul IR, Kassis I, Smolkin T, Tamir A, Sujov P. Review of 49 neonates with acquired fungal sepsis: further characterization. Pediatrics. 2001;107:61–66. doi: 10.1542/peds.107.1.61. [DOI] [PubMed] [Google Scholar]
- 130.Mestroni SC, Verna JA, Smolkin A, Bava AJ. Etiological factors of fungemia in the Hospital San Martin in La Plata. Rev Argent Microbiol. 2003;35:106–109. [PubMed] [Google Scholar]
- 131.Lopez Sastre JB, Coto Cotallo GD, Fernandez Colomer B. Neonatal invasive candidiasis: a prospective multicenter study of 118 cases. Am J Perinatol. 2003;20:153–163. doi: 10.1055/s-2003-40008. [DOI] [PubMed] [Google Scholar]
- 132.Roilides E, Farmaki E, Evdoridou J, et al. Neonatal candidiasis: analysis of epidemiology, drug susceptibility, and molecular typing of causative isolates. Eur J Clin Microbiol Infect Dis. 2004;23:745–750. doi: 10.1007/s10096-004-1210-9. [DOI] [PubMed] [Google Scholar]
- 133.Giusiano GE, Mangiaterra M, Rojas F, Gomez V. Yeasts species distribution in Neonatal Intensive Care Units in northeast Argentina. Mycoses. 2004;47:300–303. doi: 10.1111/j.1439-0507.2004.00993.x. [DOI] [PubMed] [Google Scholar]
- 134.Shetty SS, Harrison LH, Hajjeh RA, et al. Determining risk factors for candidemia among newborn infants from population-based surveillance: Baltimore, Maryland, 1998–2000. Pediatr Infect Dis J. 2005;24:601–604. doi: 10.1097/01.inf.0000168751.11375.d6. [DOI] [PubMed] [Google Scholar]
- 135.Clerihew L, Lamagni TL, Brocklehurst P, McGuire W. Invasive fungal infection in very low birthweight infants: national prospective surveillance study. Arch Dis Child Fetal Neonatal Ed. 2006;91:F188–192. doi: 10.1136/adc.2005.082024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Blyth CC, Chen SC, Slavin MA, et al. Not just little adults: candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics. 2009;123:1360–1368. doi: 10.1542/peds.2008-2055. [DOI] [PubMed] [Google Scholar]
- 137.Howell A, Isaacs D, Halliday R. Oral nystatin prophylaxis and neonatal fungal infections. Arch Dis Child Fetal Neonatal Ed. 2009;94:F429–433. doi: 10.1136/adc.2008.157123. [DOI] [PubMed] [Google Scholar]
- 138.Al-Sweih N, Khan Z, Khan S, Devarajan LV. Neonatal candidaemia in Kuwait: a 12-year study of risk factors, species spectrum and antifungal susceptibility. Mycoses. 2009;52:518–523. doi: 10.1111/j.1439-0507.2008.01637.x. [DOI] [PubMed] [Google Scholar]
- 139.Montagna MT, Lovero G, De Giglio O, et al. Invasive fungal infections in neonatal intensive care units of Southern Italy: a multicentre regional active surveillance (AURORA project) J Prev Med Hyg. 2010;51:125–130. [PubMed] [Google Scholar]
- 140.Kristof K, Janik L, Komka K, et al. Clinical microbiology of neonatal candidiasis in Hungary. Acta Microbiol Immunol Hung. 2010;57:407–417. doi: 10.1556/AMicr.57.2010.4.7. [DOI] [PubMed] [Google Scholar]
- 141.Altuncu E, Bilgen H, Cerikcioglu N, et al. Neonatal Candida infections and the antifungal susceptibilities of the related Candida species. Mikrobiyol Bul. 2010;44:593–603. [PubMed] [Google Scholar]
- 142.Peman J, Canton E, Linares-Sicilia MJ, et al. Epidemiology and antifungal susceptibility of bloodstream fungal isolates in pediatric patients: a Spanish multicenter prospective survey. J Clin Microbiol. 2011;49:4158–4163. doi: 10.1128/JCM.05474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Viudes A, Peman J, Canton E, et al. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur J Clin Microbiol Infect Dis. 2002;21:767–774. doi: 10.1007/s10096-002-0822-1. [DOI] [PubMed] [Google Scholar]
- 144.Frezza S, Maggio L, De Carolis MP, et al. Risk factors for pulmonary candidiasis in preterm infants with a birth weight of less than 1250 g. Eur J Pediatr. 2005;164:88–92. doi: 10.1007/s00431-004-1571-1. [DOI] [PubMed] [Google Scholar]
- 145.Rodriguez D, Almirante B, Park BJ, et al. Candidemia in neonatal intensive care units: Barcelona, Spain. Pediatr Infect Dis J. 2006;25:224–229. doi: 10.1097/01.inf.0000202127.43695.06. [DOI] [PubMed] [Google Scholar]