Abstract
Importance
Controversy remains about whether depression can be successfully managed after acute coronary syndrome (ACS) and the costs and benefits of doing so.
Objective
To determine the effects of providing post-ACS depression care on depressive symptoms and health care costs.
Design, Setting, and Participants
We performed a multicenter randomized controlled trial with 150 patients with elevated depressive symptoms (Beck Depression Inventory [BDI] score ≥10) 2 to 6 months after an ACS. Patients were recruited from 2 private and 5 academic ambulatory centers across the United States between March 18, 2010, and January 9, 2012.
Intervention(s)
Patients were randomized to 6 months of centralized depression care (patient preference for problem-solving treatment given via telephone or the Internet, pharmacotherapy, both, or neither), stepped every 6 to 8 weeks, (active treatment group; n=73) or to locally determined depression care after physician notification about the patient’s depressive symptoms (usual care group; n=77).
Main Outcome Measure(s)
Change in depressive symptoms during 6 months and total health care costs.
Results
Depressive symptoms decreased significantly more in the active treatment group than in the usual care group (differential change between groups, −3.5 BDI points; 95% CI, −6.1 to −0.7; P = .01). Although mental health care estimated costs were higher for active treatment than for usual care, overall health care estimated costs were not significantly different (difference adjusting for confounding, −$325; 95% CI, −$2639 to $1989; P=.78).
Conclusions
For patients with post-ACS depression, active treatment had a substantial beneficial effect on depressive symptoms. This kind of depression care is feasible, effective, and may be cost-neutral within 6 months; therefore, it should be tested in a large phase 3 pragmatic trial.
Trial Registration
clinicaltrials.gov Identifier: NCT01032018
Depression will soon be the leading cause of years lived with disability worldwide1 and contributes even more to disability when comorbid with a chronic medical disorder.2,3 Every year, approximately 1.2 million Americans survive an acute coronary syndrome (ACS) event,4 and many have clinically significant and persistent depression.5,6 Patients with post-ACS depression have significantly more ambulatory medical appointments, emergency department visits, and higher health care costs than similar patients without depression.7,8 Post-ACS depression is also associated with a 150% increased risk of ACS recurrence9 and a 100% increase in the relative risk of all-cause mortality,2,10,11 and persistent depression after an ACS event is associated with an even higher morbidity and mortality risk.3,12 Reducing persistent post-ACS depression is therefore an important public health objective.
Despite its importance, routine management of post-ACS depression remains poor12,13 because of inefficiencies in depression screening,12 lack of effective administration of depression treatment,12 weak depression treatment effects, and limited treatment options if initial efforts fail.14 Published trials have yet to demonstrate clinically significant depressive symptom reduction, have not tested strategies that would be feasible in clinical practice, are not cost-effective, and do not incorporate the preferences of ACS patients for either psychotherapy or psychotropic medications into their treatment algorithms.15
The Comparison of Depression Interventions after Acute Coronary Syndrome (CODIACS) Vanguard trial was designed to determine the feasibility, efficacy, and costs of a centralized, stepped, patient preference–based depression care system for ACS patients. This system provides depression treatment several months after an ACS, when most transient depressive reactions to ACS have spontaneously remitted but prognostic risk of ongoing depression symptoms is still high. It follows a treatment strategy that incorporates patient preference, adjusts treatment in a stepped manner as symptoms require, and centralizes the provision of care by highly trained depression specialists. We hypothesized that this approach would be cost-effective and produce larger reductions in depression than ad lib or usual care provided in the patient’s community.
METHODS
SETTING AND STUDY PARTICIPANTS
Briefly, this study was a feasibility, parallel-group, comparative effectiveness randomized controlled trial (RCT), with masked outcome assessments, in patients with persistent depressive symptoms following an ACS. The study tested whether centralized, patient preference–based depression care is cost-effective and results in larger depressive symptom reductions compared with locally administered, ad lib depression care. Participants were recruited from sites connected with 5 field centers (Columbia University, New York, New York; Washington University, St Louis, Missouri; University of Pennsylvania, Philadelphia; Emory University, Atlanta, Georgia; and Yale University, New Haven, Connecticut) from March 18, 2010, to January 9, 2012. To be eligible, participants had to demonstrate elevated depressive symptoms on the Beck Depression Inventory I (BDI)16 (BDI score ≥10 on 2 screening occasions or >15 on 1 occasion) 2 to 6 months after hospitalization for an ACS (ie, unstable angina or myocardial infarction) defined by standard criteria,17,18 be at least 35 years old, and be fluent in English or Spanish. Exclusion criteria were set for safety, retention, or intervention futility reasons (see Whang et al19 for details). The CODIACS Vanguard evaluated different recruitment strategies at different sites; details about each site’s recruitment strategy and our conclusions about the most efficient recruiting strategy can be found elsewhere.20 Briefly, automated searches of electronic medical records were conducted at most sites to identify patients who met the trial’s ACS and other medical criteria. Potential participants were then approached by a research coordinator or physician for further screening. Institutional review board–approved, written informed consent for participation was obtained before the screening interview and before randomization.
RANDOMIZATION
Participants were randomized in a 1:1 ratio according to a computer-generated, permuted-block randomization scheme, stratified by site and current antidepressant use, that local research assistants accessed by telephone. Patients were randomized to receive 6 months of centralized, stepped, patient preference–based treatment (active treatment group) or locally administered, ad lib depression care (usual care group). If randomized to the active treatment group, an unmasked research assistant met with the participant to discuss the relative benefits and risks of psychotherapy and medication use, and the participant’s initial choice was then provided.
OUTCOME MEASURES AND MASKING
Research assistants who were masked to randomization administered the BDI and ascertained adverse events, mental health and hospitalization information, and medication use at baseline and 6 months during in-person interviews and at 2 and 4 months by telephone. Patients and clinicians were not masked to group status.
TREATMENT GROUPS
The active treatment was provided by a team of professionals, including a centralized problem-solving treatment (PST) therapist, centralized psychiatry and clinical psychology supervisors, and a local study physician or advanced practice registered nurse responsible for prescribing and managing antidepressant medications. The team met weekly to discuss clinical issues, treatment quality reviews, and stepped care decisions for the active treatment participants. The Patient Health Questionnaire 9 (PHQ-9)21 was administered to participants at each study treatment session to monitor depressive symptoms and determine symptom improvement relative to baseline. If the minimum prespecified improvement criterion (see Whang et al19 for details) had not been achieved during the step period (6–8 weeks), the team offered suggestions for subsequent treatment to the clinician, and additional or alternative treatments were subsequently initiated with patient agreement. Additional treatment steps were made every 6 to 8 weeks with the aim of achieving the criterion of depression treatment success (PHQ-9 score ≤3 for 2 consecutive weeks) by the end of the intervention. Depression symptom monitoring and maintenance therapy continued on a prespecified schedule (weekly, then monthly) for the 6-month duration of the trial for those in the active treatment group who met the depression treatment success criteria.
In the intervention arm, participants chose PST, medication, both, or neither. PST is an easily disseminated, manualized, problem-focused form of cognitive behavior therapy that teaches patients how to systematically solve self-identified psychosocial problems that can trigger and perpetuate depression.22 Initially, PST was administered over the Internet via interactive video calls between the recruiting site and the coordinating center, with subsequent sessions provided by video calls or telephone, either at the clinic or at the home, depending on patient preference.23
Participants who chose pharmacotherapy were interviewed by the local study physician or nurse practitioner, and the participant and local provider agreed on the appropriate medication (sertraline, citalopram, or bupropion) and dosage based on the participant’s prior medication history and current symptoms. Medication dosing followed standard clinical practice.19 Participants were initially seen in person by the study physician or nurse at 1- to 2-week intervals for dose titration and every 3 to 5 weeks thereafter as needed. For participants who chose pharmacotherapy but who had already been prescribed a nonstudy antidepressant, subsequent treatment was coordinated with their initial prescribing practitioner.
In the Usual care condition, the participant’s primary care physician and/or cardiologist was informed by letter about the patient’s participation in the trial and his/her depressive symptom level. The participant was free to obtain any depression care from that physician or another provider.
OUTCOMES
The primary outcomes were change in BDI scores over 6 months and health care costs. To estimate costs, we searched the National Death Index, actively surveyed hospital records for ACS events, and proactively queried participants about all hospitalizations, all ambulatory care visits, cardiac procedures, and total number of hospitalization days. Participants also completed the Medical Outcomes Study 12-Item Short Form Health Survey (SF-12) and the National Institutes of Health Patient Reported Outcomes Measurement Information System (PROMIS) anxiety short form at baseline and 6 months.24,25
Statistical Analysis
The trial was powered to detect a between-group difference in the 6-month change in depression symptoms. Assuming a 5% attrition rate, we estimated that a sample of 150 patients would be needed to have 80% power to detect a clinically meaningful differential change in depression scores between groups of 0.46 SD.20
Baseline differences between the active treatment and usual care groups were evaluated using the t test for continuous variables and the χ2 test for categorical variables. Latent growth curve modeling procedures were used to generate full-information maximum-likelihood estimates of treatment effects for the entire cohort of trial participants and by subgroups. Primary analyses were conducted according to the intent-to-treat principle. Wald χ2 statistics were used to test the significance of the differential change in depressive symptoms between groups (group × time interaction).1
Logistic regression was used to determine whether exposure to active treatment was associated with increased risk of (1) depression remission (defined in the trial as a BDI score <10 at 6 months), (2) improvement in depressive symptoms (defined here as ≥0.5-SD decrease on the BDI), (3) 30 or more total depression-free days during the 6-month intervention, (4) improvement in anxiety symptoms (≥0.5 SD on the PROMIS anxiety scale), and (5) improvements in mental and physical functional status (≥0.5 SD on the SF-12 mental and physical health scales). We calculated the number needed to treat (NNT) as the inverse of the absolute risk reduction associated with active treatment vs usual care for each outcome, and for depressive symptoms we calculated a Hedges’ G effect size2.
Ambulatory care costs were based on Current Procedural Terminology codes and Medicare’s Physician Fee Schedule for non–facility-based care, mental health costs on appointment duration, hospitalization costs on Medicare diagnosis-related-group hospital payments with an adjustment for physicians’ professional fees based on length of stay,26 and antidepressant and anxiolytic medication costs on the 2010 Red Book midrange average wholesale price for generic medications.27 All costs are presented in 2011 US dollars and adjusted to the US Consumer Price Index. To determine whether group assignment predicted cost outcomes, we conducted linear regression models, adjusted for age, sex, ethnicity, race, marital status, education, baseline BDI score, type of ACS, and left ventricular ejection fraction (see Ladapo et al28 for details).3
RESULTS
STUDY PARTICIPANTS
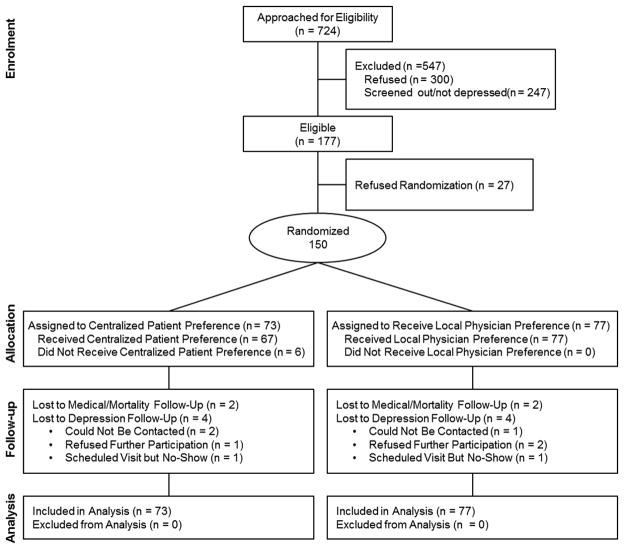
Potential participants with ACS (n = 724) were asked to participate in an eligibility interview; 177 patients (24.4%) were found eligible and 150 were enrolled and randomly allocated (Figure 1): 73 to active treatment and 77 to usual care. Two participants in each group were lost to follow-up at 6 months, and 4 from each group (5.3% total) had missing 6-month depression data. Table 1 provides baseline demographic and clinical characteristics by group.
Figure 1.
CONSORT diagram of the CODIACS Vanguard trial.
Table 1.
Characteristics of 150 Patients With Acute Coronary Syndrome and Depression Symptomsa
| Characteristic | Active Treatment, No. (%) (n=73) | Usual Care, No. (%) (n=77) | P Valueb |
|---|---|---|---|
| Mean (SD) age, y | 59.2 (9.7) | 60.0 (11.1) | .65 |
|
| |||
| Female | 30 (41.1) | 33 (42.9) | .83 |
|
| |||
| Hispanic | 16 (21.9) | 13 (16.9) | .42 |
|
| |||
| African American | 23 (31.5) | 27 (35.1) | .64 |
|
| |||
| Education | .61 | ||
| High school degree | 39 (60.9) | 46 (67.6) | |
| College degree | 21 (32.8) | 17 (25.0) | |
|
| |||
| Type of presenting acute coronary syndrome | .95 | ||
| Unstable angina | 35 (47.9) | 35 (45.5) | |
| Non–ST-segment elevated myocardial infarction | 18 (24.7) | 19 (24.7) | |
| ST-segment elevated myocardial infarction | 19 (26.0) | 21 (27.3) | |
| Bundle branch block/uncertain type of myocardial infarction | 1 (1.4) | 2 (2.6) | |
|
| |||
| Left ventricular ejection fraction <0.45c | 18 (31.6) | 13 (23.2) | .32 |
|
| |||
| Beck Depression Inventory score | 21.0 (7.6) | 20.6 (7.8) | .77 |
|
| |||
| Beck Depression Inventory score ≥16 at baseline | 52 (71.2) | 50 (64.9) | .41 |
|
| |||
| Ever prescribed antidepressant or antianxiety medicationd | 43 (58.9) | 42 (55.3) | .65 |
|
| |||
| Use of antidepressant medication(s) at time of enrollmentd | 25 (34.2) | 23 (31.1) | .68 |
Data are number (percentage) of study participants unless otherwise indicated.
P values are based on the χ2 test for categorical measures and independent sample t test for continuous measures.
Data on left ventricular ejection fraction at index hospitalization were available for only 113 patients (left ventricular ejection fraction not assessed, 36 patients; medical history form not received, 1 patient).
Findings are based on self-report data.
STUDY TREATMENT
Of the 73 active treatment patients, 41 (56.2%) initially chose PST, 9 (12.3%) chose antidepressant medication, 17 (23.3%) chose both, and 6 (8.2%) chose neither. The mean number of PST sessions for those who initially chose PST was 7.7 (range, 1–25). Of the 77 patients randomized to usual care, 9 (11.7%) received psychotherapy, and another 9 (11.7%) received antidepressants new to their treatment regimen.
ANTIDEPRESSANT AND PSYCHOTHERAPY USE BEFORE AND AFTER THE TRIAL
Of the 150 trial participants, 25 of the active treatment and 26 of the usual care participants reported that they were already receiving antidepressants at randomization; at the end of the trial, 37 of the 73 active treatment participants (50.7%) and 28 of the 77 usual care participants (36.4%) were still using antidepressants(χ2 (1) = XX, p< XX). Before randomization, 6 (8.2%) and 9 (11.7%) patients in the active treatment and usual care groups, respectively, were participating in psychotherapy; at the end of the trial, these numbers had increased to 48 (65.8%) and 14 (18.2%), respectively (χ2 (1) = XX, p< XX).
DEPRESSION OUTCOMES
The active treatment group experienced a greater reduction in BDI scores (−10.1; 95% CI, −12.0 to −8.1) than the usual care group (−6.6; 95% CI, −8.5 to −4.8), resulting in a significant differential change between groups of −3.5 BDI points (95% CI, −6.1 to −0.7; P = .01; Hedges’ G = 0.59) (Figure 3). Remission of depression (BDI score <10 at 6 months) occurred in 24 active treatment participants (47.1%) and 16 usual care participants (27.6%) (P = .04; number needed to treat = 5). No significant differences were found between groups in the proportion of participants who achieved a 0.5-SD reduction of depressive symptoms) or 30 or more depression-free days (Table 2).
Figure 3.
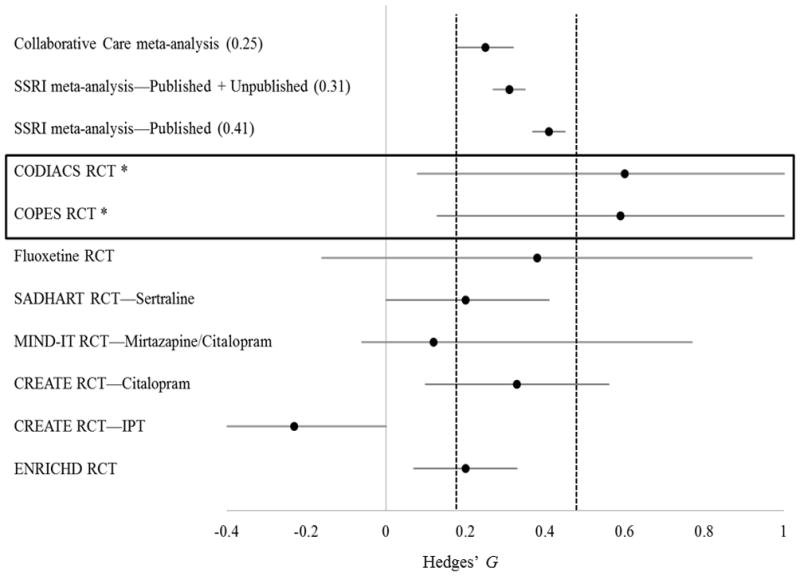
Comparison of the CODIACS Vanguard trial with other trials of depression treatments in coronary heart disease and primary care patients. *This trial included participants with elevated depressive symptoms; other trials included only patients with major depressive disorder. It is also important to note that some of these trials had more active control conditions, leading possibly to smaller depression differences between groups. Full-information maximum-likelihood estimates were used to determine intent-to-treat treatment effects; other trials may have used other estimating methods. IPT indicates interpersonal psychotherapy.
Note: a larger Hedges’ G indicates a stronger positive effect of the tested intervention.
Note: Vertical dashed lines indicate the 95% confidence intervals for the Hedges’ G estimates from the three meta-analyses.
Note: Horizontal solid line indicates the 95% confidence interval for each individual study
Table 2.
Six-Month Improvements in Mental Health, Functional Status, Health Utilities, and Clinical End Pointsa
| Variable | Active Treatment, No. (%) | Usual Care, No. (%) | RR (95% CI) | P Value | No. Needed to Treat |
|---|---|---|---|---|---|
| Depressive symptoms | |||||
| BDI score <10 at 6 months | 24/51 (47.1) | 16/58 (27.6) | 1.70 (1.04 – 2.40) | .04 | 5 |
| 0.5-SD improvement in BDI score | 38/51 (74.5) | 39/58 (67.2) | 1.11 (0.83 – 1.30) | .41 | 14 |
| ≥30 Depression-free days | 38/73 (52.1) | 37/77 (48.1) | 1.08 (0.76 – 1.40) | .62 | 25 |
| Anxiety symptoms | |||||
| 0.5-SD improvement in PROMIS anxiety scale | 36/62 (58.1) | 27/62 (43.5) | 1.34 (0.93 – 1.70) | .11 | 7 |
| Functional status and health utilities | |||||
| 0.5-SD improvement in SF-12 Mental Composite Score | 33/65 (50.8) | 33/70 (47.1) | 1.08 (0.73 – 1.42) | .67 | 27 |
| 0.5-SD improvement in SF-12 Physical Composite Score | 14/65 (21.5) | 20/70 (28.6) | 0.76 (0.39 – 1.32) | .35 | NA |
Abbreviations: BDI, Beck Depression Inventory; NA, not applicable (because the observed effect did not favor the intervention conditions); PROMIS, Reported Outcomes Measurement Information System; SF-12, Medical Outcomes Study 12-Item Short Form Health Survey.
To calculate the number needed to treat, we used only observed data; thus, sample sizes vary and are presented for each grouping.
Exploratory post hoc subgroup analyses revealed a significant sex × group × time interaction (P = .03); women’s depressive symptom scores decreased more (−6.4; 95% CI, −10.1 to −2.6) than men’s (−1.6; 95% CI, −6.7 to 3.6) in active treatment group (Supplemental Table 1). There was also a significant diabetic status × group × time interaction (P = .0499); the depressive symptom scores of patients with diabetes decreased more (−6.2; 95% CI, −10.0 to −2.3) than did those of patients without diabetes in the active treatment group (−0.9; 95% CI, −4.6 to 2.7). No other subgroup differences were found, including the comparison of those with probable mild (BDI 10–15) vs major (BDI ≥16) depression.
HEALTH CARE COSTS
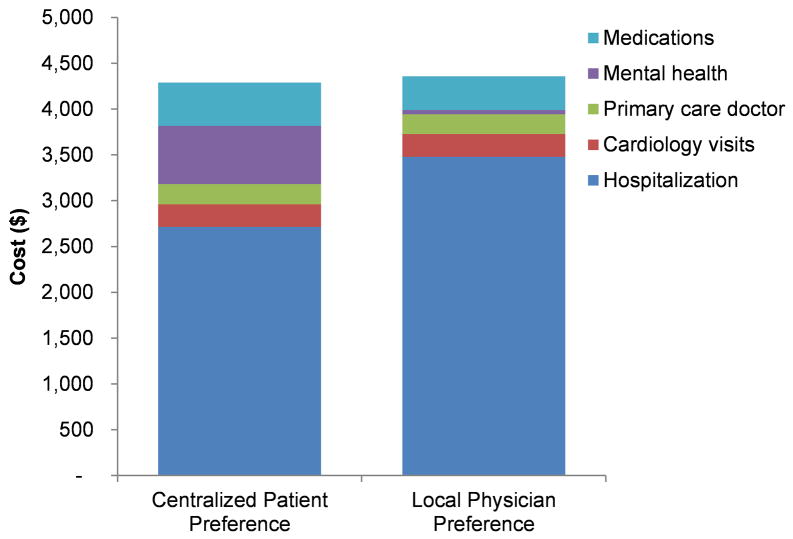
Mental health costs were significantly higher for the active treatment group than for the usual care group (adjusted change, +$687; 95% CI, $466 to $909; P<.001), while average hospital costs were lower in the intervention group (adjusted change, −$1,010; 95% CI, −$3,294 to $1,274; P=0.39). As a result of this offset, total health care costs in the study intervention group were not higher than in the comparison group (adjusted change, −$325; 95% CI, −$2639 to $1989; P=.78; Figure 2, Supplemental Table 2).
Figure 2.
Comparison of health care use costs during the 6 months between treatment groups.
DISCUSSION
The CODIACS Vanguard provides a basis for future studies to determine whether treating depression reduces mortality and cardiac event recurrence in post-ACS patients. It demonstrates that this approach yields a substantial reduction in depressive symptoms, replicating our earlier trial finding. 14 The size of the study intervention’s effect on depressive symptoms (Hedges’ G = 0.59) compares favorably with the results of interventions tested in other trials. Figure 3 compares these findings with those of a prior trial that tested a patient preference–based intervention,14 other recent depression trials in ACS patients,29–33 and meta-analyses of both psychotherapy22 and antidepressant medication trials.34
Post hoc testing of 10 subgroup differences identified two apparent subgroup effects: the study intervention had stronger effects on depression reduction in women than in men, and in patients with diabetes than in patients without diabetes. Some previous depression trials in cardiac populations found weaker treatment effects for women than for men.33,35 Additional studies would be required to confirm whether the study intervention employed here has gender-specific benefits or specific benefit for depressed post-ACS patients with comorbid diabetes.
This trial has several limitations. It enrolled patients with elevated depressive symptoms who did not necessarily meet criteria for a major depressive disorder. Some institutional review board committees consider any delay in treating diagnosed depression as unethical, and the gold standard for diagnosis of major depressive disorder requires a lengthy psychiatric interview conducted by a trained expert. Both these considerations impede the conduct of a pragmatic RCT for post-ACS depression. In addition, even patients with subsyndromal depressive symptoms are at elevated risk for adverse medical outcomes and experience impaired quality of life, suggesting that such patients may benefit from treatment. The usual care condition did not control for the amount of clinical attention participants received. Evidence about the usefulness of any management approach is cumulative; other control conditions should be considered for future trials. Our estimate of costs must be viewed as very preliminary; although our point estimate indicated a possible cost saving associated with the study intervention, the effect was not significant and the confidence interval was quite wide. In addition, we likely underestimated intervention costs because we did not account for some aspects of care coordination. Finally, central coordination of phone and internet interventions may be the treatment of the future, but may be difficult to implement currently, given the structure of health care treatment of patients with ACS.
The intriguing hypothesis that depression is causally implicated in ACS recurrence and death36 has never been adequately tested despite decades of observational prospective research reporting that elevated depressive symptoms are strongly associated with these outcomes. Skepticism concerning this causal hypothesis12 has persisted and contributes to uncertainty among clinicians about whether to treat post-ACS depression. To our knowledge, no RCTs of depression treatment have definitively demonstrated a reduction in cardiovascular disease recurrence or death. Most widely used treatments have limited efficacy for depression reduction in post-ACS patients, impeding a proper test of the causal hypothesis. Depressive symptoms remain on par with other established coronary heart disease risk factors for strength of association with ACS recurrence and mortality risk.37 Cardiologists, primary care physicians, and family physicians are being urged to screen for depression, without sufficient trial data to guide subsequent treatment. Advisories and guidelines from the American Heart Association,38,39 American Academy of Family Practitioners,40 European professional cardiology societies,41 and the British health care system42 recommend depression screening in post-ACS patients and referral for treatment if depression is found. However, no cost-effectiveness RCT data are available to inform this large, potentially expensive guideline recommendation. There has been only one sufficiently powered RCT on which to base treatment decisions,33 which is clearly an inadequate basis for empirically supported practice.
We now have new signals of a substantial and possibly cost-neutral depression treatment benefit from this and other RCTs20,43–46 on which to base a definitive ACS depression trial. A large phase 3 trial would inform evidence-based depression treatment guidelines for patients with an ACS, and even has the tantalizing possibility of answering the important question of whether treating depression in ACS patients lowers mortality and recurrence rates.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by grant 5RC2HL101663, HL-088117, HL-84034 from the National Institutes of Health, Bethesda, MD. Supported in part by Columbia University’s CTSA grant No. UL1TR000040 from NCATS/NIH.
Footnotes
Because the distribution of BDI scores was positively skewed, all analyses were conducted using square-root transformed BDI scores, which were then back-transformed for ease of interpretation.
The effect size allows comparisons of the effect of an intervention across trials when different depression measures are used.
Cost data were positively skewed but we analyzed them with parametric models without a log transformation to facilitate a more natural interpretation, and because our inferences did not significantly change when a log-linear multivariate regression model was used.
Financial Disclosure: This trial was funded by the National Heart, Lung, and Blood Institute, which had no formal role in the design, conduct, data collection, management, analysis, interpretation, preparation, review, or approval of the manuscript; the views expressed herein are those of the authors. All authors declare that they have no relevant conflicts of interest.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 3.Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105(9):1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 5.Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121(11 Suppl 2):S20–27. doi: 10.1016/j.amjmed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Bush DE, Ziegelstein RC, Patel UV, et al. Post-myocardial Infarction Depression. Washington, DC: Agency for Healthcare Research and Quality; 2005. p. 123. [Google Scholar]
- 7.Egede LE. Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry. 2007;29(5):409–416. doi: 10.1016/j.genhosppsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Whooley MA, Kiefe CI, Chesney MA, Markovitz JH, Matthews K, Hulley SB. Depressive symptoms, unemployment, and loss of income: The CARDIA Study. Arch Intern Med. 2002;162(22):2614–2620. doi: 10.1001/archinte.162.22.2614. [DOI] [PubMed] [Google Scholar]
- 9.van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66(6):814–822. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. European Heart Journal. 2006;27(23):2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 11.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290(2):215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thombs BD, de Jonge P, Coyne JC, et al. Depression Screening and Patient Outcomes in Cardiovascular Care: A Systematic Review. JAMA. 2008;300(18):2161–2171. doi: 10.1001/jama.2008.667. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez HM, Vega WA, Williams DR, Tarraf W, West BT, Neighbors HW. Depression care in the United States: too little for too few. Arch Gen Psychiatry. 2010;67(1):37–46. doi: 10.1001/archgenpsychiatry.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson KW, Rieckmann N, Clemow L, et al. Stepped depression care for Acute Coronary Syndrome patients with persistent depression: Coronary Psychosocial Evaluation Studies (COPES) randomized controlled trial. Arch Intern Med. 2009;170(7):8. doi: 10.1001/archinternmed.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burg MM, Rieckmann N, Clemow L, Medina V, Schwartz J, Davidson KW. Treatment preferences among depressed patients after acute coronary syndrome: the COPES observational cohort. Psychother Psychosom. 2011;80(6):380–382. doi: 10.1159/000323615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 17.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 18.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Whang W, Burg MM, Carney RM, et al. Design and baseline data from the vanguard of the comparison of depression interventions after acute coronary syndrome (CODIACS) randomized controlled trial. Contemp Clin Trials. 2012;33(5):1003–1010. doi: 10.1016/j.cct.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson KW, Rieckmann N, Clemow L, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med. 2010;170(7):600–608. doi: 10.1001/archinternmed.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative Care for Depression: A Cumulative Meta-analysis and Review of Longer-term Outcomes. Arch Intern Med. 2006;166(21):2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 23.Simon GE, Ludman EJ, Tutty S, Operskalski B, Von Korff M. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment: a randomized controlled trial. JAMA. 2004;292(8):935–942. doi: 10.1001/jama.292.8.935. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JA, Maddigan SL. Performance of the RAND-12 and SF-12 summary scores in type 2 diabetes. Qual Life Res. 2004;13(2):449–456. doi: 10.1023/B:QURE.0000018494.72748.cf. [DOI] [PubMed] [Google Scholar]
- 25.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Institute of Medicine. [Accessed January 17, 2012, 2011];New Data on Geographic Variation: Report 1: Single HRR Comparison to National Data - Demographic, Cost, Utilization, and Quality. 2011 http://iom.edu/Activities/HealthServices/GeographicVariation/Data-Resources.aspx.
- 27.Thomson Corporation. Red Book 2010: Pharmacy’s Fundamental Reference. Montvale, NJ: Thomson PDR; 2010. [Google Scholar]
- 28.Ladapo JA, Shaffer JA, Fan Y, Ye S, Davidson KW. Cost-effectiveness of enhanced depression care after acute coronary syndrome: Results from the coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med. doi: 10.1001/archinternmed.2012.4448. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strik J, Honig A, Lousberg R, et al. Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: Findings from a double-blind, placebo-controlled trial. Psychosom Med. 2000;62(6):783–789. doi: 10.1097/00006842-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 31.van Melle JP, de Jonge P, Ormel J, et al. Relationship between left ventricular dysfunction and depression following myocardial infarction: data from the MIND-IT. Eur Heart J. 2005;26(24):2650–2656. doi: 10.1093/eurheartj/ehi480. [DOI] [PubMed] [Google Scholar]
- 32.Lesperance F, Frasure-Smith N, Koszycki D, et al. Effects of Citalopram and Interpersonal Psychotherapy on Depression in Patients With Coronary Artery Disease: The Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) Trial. JAMA. 2007;297(4):367–379. doi: 10.1001/jama.297.4.367. [DOI] [PubMed] [Google Scholar]
- 33.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 34.Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358(3):252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- 35.Katon W, Russo J, Lin EHB, et al. Cost-effectiveness of a Multicondition Collaborative Care Intervention: A Randomized Controlled Trial. Arch Gen Psychiatry. 2012;69(5):506–514. doi: 10.1001/archgenpsychiatry.2011.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rollman BL, Belnap BH, LeMenager MS, et al. Telephone-Delivered Collaborative Care for Treating Post-CABG Depression: A Randomized Controlled Trial. JAMA. 2009;302(19):2095–2103. doi: 10.1001/jama.2009.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45(5):637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. Depression and Coronary Heart Disease. Recommendations for Screening, Referral, and Treatment. A Science Advisory From the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research. Circulation. 2008;118(17):1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 39.Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol. 2007;49(11):1230–1250. doi: 10.1016/j.jacc.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Post-Myocardial Infarction Depression Clinical Practice Guideline Panel. AAFP guideline for the detection and management of post-myocardial infarction depression. Ann Fam Med. 2009;7(1):71–79. doi: 10.1370/afm.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J. 2007;28(19):2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 42.National Institute for Health and Clinical Excellence. Depression in Adults with Chronic Physical Health Problems. London: National Institute for Health and Clinical Excellence; 2009. [Google Scholar]
- 43.Freedland KE, Skala JA, Carney RM, et al. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Arch Gen Psychiatry. 2009;66(4):387–396. doi: 10.1001/archgenpsychiatry.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 45.Katon WJ, Lin EHB, Von Korff M, et al. Collaborative Care for Patients with Depression and Chronic Illnesses. N Engl J Med. 2010;363(27):2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gulliksson M, Burell G, Vessby B, Lundin L, Toss H, Svardsudd K. Randomized Controlled Trial of Cognitive Behavioral Therapy vs Standard Treatment to Prevent Recurrent Cardiovascular Events in Patients With Coronary Heart Disease: Secondary Prevention in Uppsala Primary Health Care Project (SUPRIM) Arch Intern Med. 2011;171(2):134–140. doi: 10.1001/archinternmed.2010.510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.