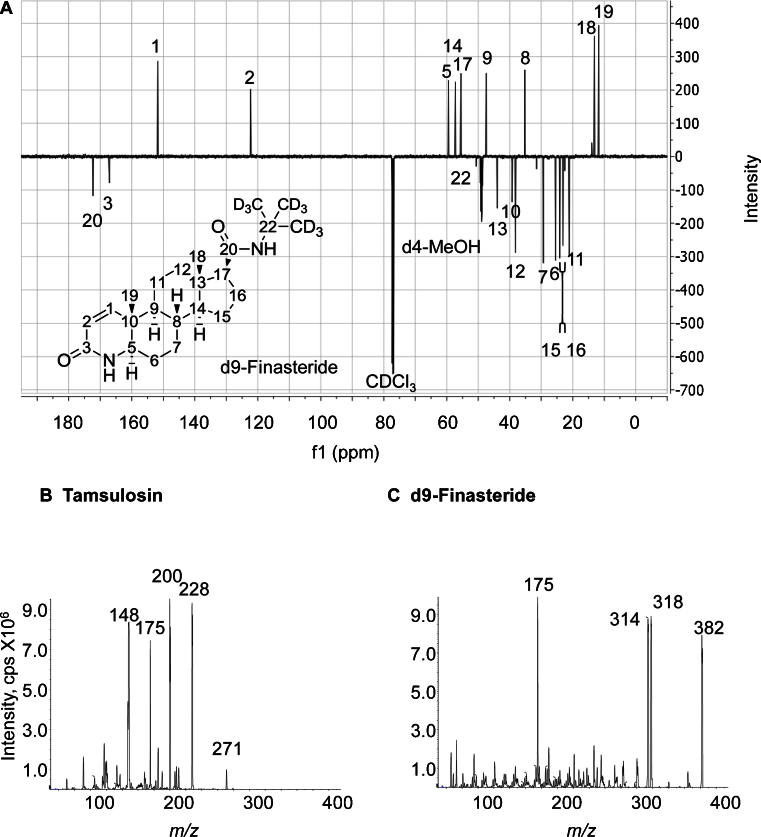
Fig. 2.
(A) d9-Finasteride DQ135 13C NMR (126 MHz, CDCl3) δ = C-20: 172.29, C-3: 167.09, C-1: 151.71, C-2: 122.30, C-5: 59.56, C-14: 57.29, C-17: 55.48, C-22: 50.63, C-9: 47.47, C-13: 43.97, C-10: 39.27, C-12: 38.16, C-8: 35.22, C-7: 29.33, C-6: 25.55, C-15: 24.15, C-16: 23.12, C-11: 21.13, C-18: 13.13, C-19: 11.76. Assignation was carried out based on previously published data [16]. A reference sample of finasteride scanned on the same instrument gave the same signals, apart from the presence of the intense tert-butyl CH3 signal at 28.7 ppm. cps, counts per second. (B) Product ion spectra for protonated tamsulosin in electrospray ionisation mode, with m/z 228 and 200 selected as quantifier and qualifier ions respectively. (C) Product ion spectra for protonated d9-finasteride in electrospray ionisation mode, with m/z 318 and 314 selected as quantifier and qualifier ions respectively. Product ion spectra for both tamsulosin and d9-finasteride were collected under the following conditions: declustering potential 119 V, collision energy 35 V, cell exit potential 16 V.