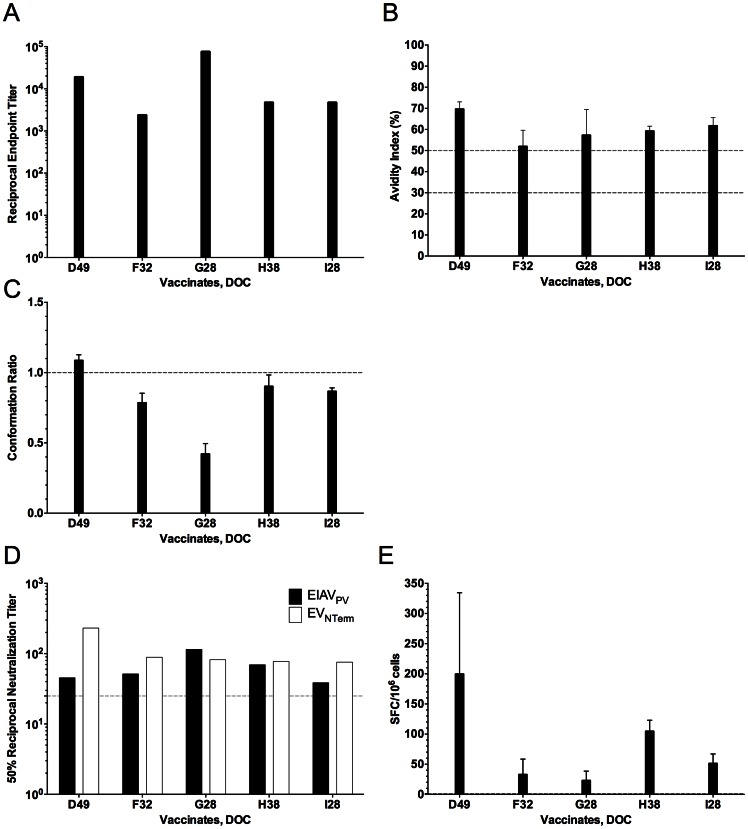
Figure 5. Day of challenge humoral and cellular immune responses of EIAVD9 vaccinates.
Characterization of the quantitative and qualitative properties of induced EIAV envelope-specific humoral and cellular responses on the day of challenge were conducted in ConA serological ELISA assays of serum antibody (A) endpoint titer, (B) avidity, and (C) conformational dependence; (D) 50% serum neutralization titer determinations, and (E) INF-γ ELISpot of PBMC, all as described in Materials and Methods. (A) Mean serum antibody titers are presented as the log10 of the highest reciprocal dilution yielding reactivity two standard deviations above background. (B) Mean avidity index measurements are presented as percentages of the antibody-antigen complexes resistant to disruption with 8 M urea. (C) Mean conformation dependence values are calculated as the ratio of serum antibody reactivity with native envelope compared to denatured envelope antigen. Conformation ratios greater than 1.0 indicate predominant antibody specificity for conformational determinants, while ratios less than 1.0 indicate predominant antibody specificity for linear envelope determinants. (D) The mean reciprocal dilutions of serum from vaccinated horses which neutralized 50% of input EIAVPV or EVNTerm, as measured in an infectious center assay. The line (–-) denotes the cut off (≥25) value for valid 50% neutralization titers. (E) EIAV Env-specific cellular activity measured as the mean INF-γ ELISpot analysis of EIAV gp90 peptide stimulation of PBMC from vaccinated horses. SFC, Spot-forming Cells.