Abstract
Isolating processes within the brain that are specific to human behavior is a key goal for social neuroscience. The current research was an attempt to test whether recent findings of enhanced negative ERPs in response to unexpected human gaze are unique to eye gaze stimuli by comparing the effects of gaze cues with the effects of an arrow cue. ERPs were recorded while participants (N = 30) observed a virtual actor or an arrow that gazed (or pointed) either toward (object congruent) or away from (object incongruent) a flashing checkerboard. An enhanced negative ERP (N300) in response to object incongruent compared to object congruent trials was recorded for both eye gaze and arrow stimuli. The findings are interpreted as reflecting a domain general mechanism for detecting unexpected events.
Keywords: eye gaze, face perception, theory of mind, ERP, high-density ERP
INTRODUCTION
Often we do more than simply observe another person move, we predict their intention in relation to the occurrence of some event. We take an intentional stance (Dennett, 1987) with respect to the behavior of the person—we expect them to behave in a certain way. In human ancestral environments, the ability to predict the behavior of others may have been adaptive (e.g. during interpersonal conflict) and consequently, specialized neural mechanisms may have evolved to perform such a function. Researchers have adopted a number of approaches to help identify the neural mechanisms responsible for predicting the behavior of others. One approach has been to study the neural process responsible for the processing trait-related information (see for example; Bartholow et al., 2001; Mitchell et al., 2005; Van Duynslaeger et al., 2007) whereby observers expect individuals or groups of individuals to behave in certain ways (e.g. acting in a friendly manner based on an impression made earlier).
A second approach (see for example; Pelphrey et al., 2003) adopted here, has a more narrow focus on movement-related expectancies specifically, and aims to identify the neural processes that are responsible for responding to unexpected movement (e.g. looking left when looking right is expected). Movement-related expectancies for eye gaze in particular, may play a key functional role in social interaction. For example, if we see a person looking toward a speaker during a face-to-face encounter we might think that they intend to listen to, and perhaps communicate with, the speaker. The significance of gaze for social interaction finds support from a number of sources including deficits in the use of eye gaze information in individuals with an impaired ability to read the mind of others (in autism spectrum disorders; Baron-Cohen, 1995). Given the likely functional value of eye gaze direction for predicting the intentions of others, recent brain activation studies have attempted to describe the neural correlates of responses to violations of movement-related expectation from eye gaze (e.g. Pelphrey et al., 2003) and how such processes are affected in autism (Pelphrey et al., 2005a). Our goal is to replicate one such pattern—electrophysiological evidence for enhanced negativity to unexpected eye gaze direction (Senju et al., 2006)—and to test the extent to which such a pattern reflects specific processes for unexpected gaze direction or more general processes for unexpected direction (arrow) cues.
Brain activation to unexpected movement
Numerous findings support the idea that the superior temporal sulcus (STS) and more specifically, the posterior region of the STS (pSTS) is a key neural region within a network of structures that are specialized for the processing of human non-verbal cues (for a review see; Puce and Perrett, 2003) including human motion from point light displays (e.g. Saygin et al., 2004), body motion (e.g. Morris et al., 2006) and motion of the hand (Pelphrey et al., 2005a) and face (Puce et al., 1998; Pelphrey et al., 2005b). Further evidence supports the idea that the pSTS has a more specific role in inferring intentions from human action. For example, Pelphrey et al. (2003) examined brain activation in participants who observed a virtual actor either looking towards (congruent trials) or away from (incongruent trials) a location that had recently contained a small checkerboard. Incongruent trials were conceptualized as requiring the observer to reformulate their initial expectation (that the observer would look toward the location where the checkerboard had been). Increased activation on incongruent trials relative to congruent trials was conceptualized as reflecting expectation of behavior from movement. Although both trials elicited activity in the pSTS, the activity was significantly greater on incongruent compared to congruent trials. These effects have been replicated in children (Mosconi et al., 2005) and also, for other types of violations of expectation from other types of human action (Grezes et al., 2004; Pelphrey et al., 2004; Morris, et al., 2005; Saxe et al., 2004) including the violation of rational actions (Brass et al., 2007; Jastorff et al., 2011) and the violation of expectation from observation of emotional reactions (Wyk et al., 2009).
Research studies that have compared individuals with autism with control participants adds further support to the hypothesis that the STS is responsible for forming expectations based on observation of movement. Individuals with autism are typically able to make perceptual judgments based on eye gaze cues but are impaired in their ability to infer intent and mental states from eye gaze (Baron-Cohen et al., 1985, 1999; Leekam et al., 1998, 2000). Using the same task as used previously, Pelphrey et al. (2005a) found that the pSTS was activated in all participants. However, controls but not individuals with autism showed the differential activation recorded for incongruent compared to congruent eye gaze trials. Taken together with the findings from non-clinically disordered populations these findings corroborate the idea that regions of the STS are specialized for inferring intent from human action.
The effects reported by Pelphrey et al. (2003) in non-clinically disordered individuals have been extended in a recent study (Senju et al., 2006) that recorded ERPs in adults and infants. In the latter study, the target object (a flickering checkerboard) was removed at the same time as the onset of the gaze shift to reduce the likelihood of recording gaze orienting effects (e.g. Schuller and Rossion, 2001). In adults, there was an enhanced posterior occipito-temporal component (N330) for object-incongruent compared to object-congruent gaze shifts. In infants, a similar but somewhat earlier negative component (N290) was recorded for object-incongruent relative to object-congruent gaze shifts. Also, infants but not adults showed larger (negative) amplitudes for object-congruent shifts in anterior brain regions. Although no attempt was made to localize the effects, the findings were interpreted in conjunction with fMRI research (e.g. Pelphrey et al., 2003) as indicating the operation of the STS in response to violations of expectation for human action.
To what extent are the effects reported by Senju et al. (2006) specific to expected movement of the eyes? An unexplored possibility is that these effects are not the result of expectations of movement specifically, but rather reflect the operation of a more general system for processing unexpected, low frequency events that might include gazing at an unexpected location. In other words, it is unclear whether the effects are unique to human cues as might be expected for a specialized process. What is lacking is a comparison of such effects with stimuli that are not part of the human body. The novel approach taken here is to compare the effects due to uniquely human cues that indicate the location of an event (e.g. eye gaze cues) with non-human directional cues such as arrows.
Although both eye gaze and arrows are capable of producing involuntary shifts of attention (Ristic et al., 2002; Tipples, 2002) recent research has shown that the effects of the two types of cue on attentional orienting can be dissociated at the neural level. Reorienting to gaze and arrows activate distinct brain areas (Greene et al., 2009; Engell et al., 2010) and moreover, orienting to gaze and arrows produce distinct patterns of neural activation in autism spectrum disorders (Greene et al., 2011). Furthermore, tasks designed to measure thoughts about other people’s minds (or Theory of Mind) can be further dissociated from the neural areas responsible for reorienting attention to non-social cues (Scholz et al., 2009; see also; Mitchell, 2007) and therefore, one explanation for distinct areas of activation when reorienting to eye gaze cues is that theory of mind mechanisms contribute to orienting to eye gaze; a possibility that garners further support from recent behavioral data (Teufel et al., 2010). Overall, these studies support that distinct neural process may underpin responses to eye gaze stimuli on tasks that are thought to engage thinking about other people’s thoughts. In light of such findings, we tested whether the effects reported by Senju et al. (2006) are specific to gaze cues by comparing the effects of unexpected (or object incongruent) eye gaze direction with the effect of unexpected arrow direction.
With this goal in mind, we recorded ERPs while participants viewed arrows and eye gaze shifts that were either congruent or incongruent with location of a flickering checkerboard. If previous effects are unique to human movement then the enhanced negative amplitudes recorded at posterior electrodes in response to breaches in expectation of direction will be specific to eye gaze cues. If such effects reflect domain-general processes then such effects will occur for both gaze and arrow cues. To preempt—enhanced negativity in posterior networks was recorded for both eye gaze and arrow cues.
METHODS
Thirty-two healthy right-handed volunteers participated in this study. Thirty of them (18 female, 12 male; age range: 18–34 years old; average age: 20 years) provided an adequate number of artifact-free ERP trials and were included in the analysis. Two participants were removed from the data analyses due to a high number (>20%) of artifacts. All participants had normal or corrected to normal visual acuity. Participants provided written informed consent.
Stimuli
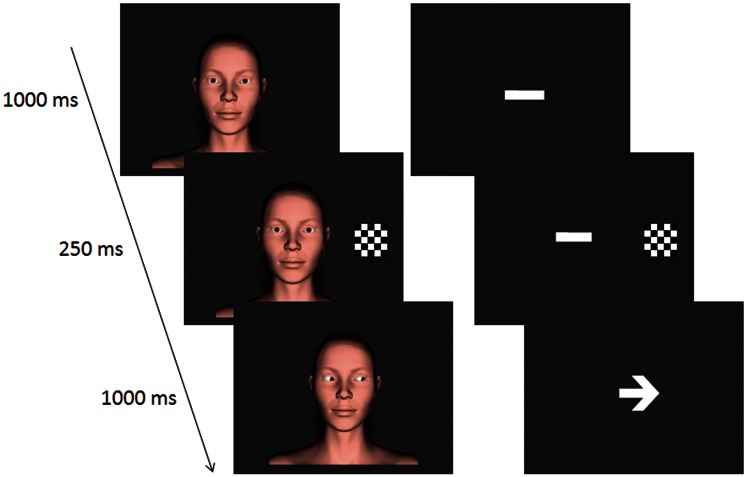
Examples of the stimuli are displayed in Figure 1. On eye gaze trials a face (subtending 5.73° wide and 12.37° high), was used as the stimulus. The face was created by a commercial company (DAZ Productions, Inc., Draper UT) for use with the software program, Poser 5.0® (Curious Labs Inc., Santa Cruz, CA, USA). On arrow trials a white line (subtending 1.91° wide and 1.43° high) was used as the initial fixation stimulus
Fig. 1.
An example of a trial sequence for an eye congruent (left) and an arrow congruent (right) trial.
Procedure
Each trial began with the onset of a face looking forward or a white line. Example trial sequences are displayed in Figure 1. All participants viewed trials which involved either eye-gaze cues or arrow cues. Participants were instructed to keep their eyes fixated on the bridge of the nose or the centre of the white line. After 1000 ms, a flashing checkerboard cue appeared either to the left or right of the face (or line) for 250 ms. Following the disappearance of the checkerboard, either the eyes changed position or an arrow head appeared. The eyes or the arrow were directed equally often towards (congruent condition) or away from (incongruent) the location previously occupied by the flashing checkerboard cue. Participants were asked to fixate on the face and press the Z key the keyboard if the arrow or gaze direction matched the location of the checkerboard or the M key if the cue pointed away from the location of the checkerboard. The averted eyes or arrow appeared for 1000 ms before the start of a new trial (and reappearance of the fixation stimuli). EEG was recorded across eight blocks of trials lasting ∼45 min. Within each block, each balanced combination of stimulus type (eye, arrow), checkerboard location (left, right) and cue direction (left, right) was displayed eight times. Within each block, trials were presented in a new random order for each participant.
EEG recording and analyses
EEG was recorded from 64 channel Easycap (using standard 10–10 electrode placement) and referenced to the nose. Electrode impedances were kept <5 kΩ for the reference and 5 kΩ for the EOG electrodes and 10 kΩ for the remaining electrodes. Data were recorded continuously at a sampling rate of 500 Hz, with a bandpass filter of 0.05–100 Hz and a 50-Hz notch filter. Horizontal EOG was recorded from electrodes placed near the outer canthi of the eye and vertical EOG was recorded from electrodes placed above and below the left eye.
Offline, a 0.5- to 40-Hz band-pass filter was applied to the data. Epochs were centered on the onset of the eye gaze or arrowhead direction, and baselined to the period from −450 to −250 ms prior to the onset of the checkerboard. Epoch length was 1450 ms (beginning 450 before trigger). Artifact rejection of ±75 µV was applied; further visual inspection of the data was conducted to remove any remaining contaminated trials. Data for each condition were averaged to produce ERP waveforms. These waveforms were then combined to produce group-averaged data.
Following Senju et al. (2006), five regions of interest were selected for the analyses. In the current study, the recording montage differed slightly from that used by Senju et al (2006). We therefore selected channels PO3, PO4, POZ as corresponding to Senju et al.'s (2006) midline Lower-Occipital region, left: T7, TP7, P7; right: T8, TP8, P8 as corresponding to Left and Right Lateral regions, and left: CP5, CP3, P5, P3, PO7, PO5; right: CP4, CP6, P4, P6, PO4, PO6, as corresponding to Left and Right Semi-Medial regions. Data were averaged across these electrode groupings for the region of interest analyses.
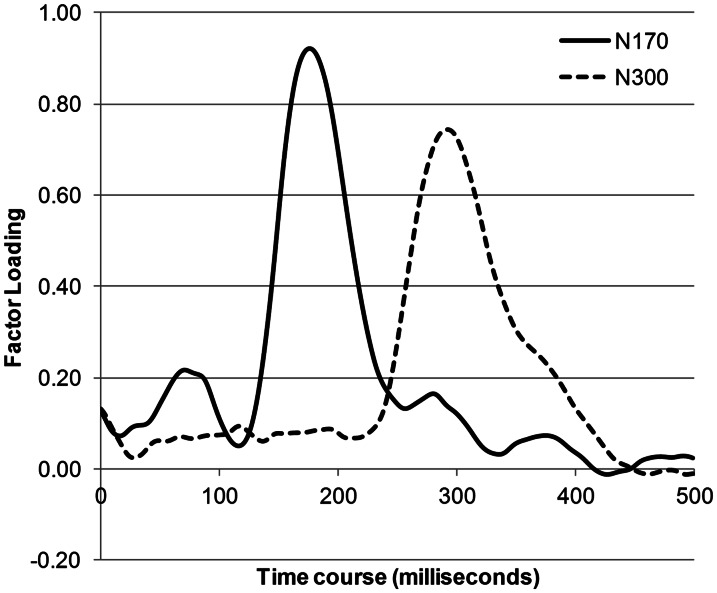
Two complementary analysis strategies were used to help identify independent ERPs of interest. First, grand-averaged data, as well as individuals’ average ERPs, were visually inspected and three time windows were chosen to capture the components sensitive to object congruency: P120 (100–150 ms) at the Lower-Occipital region, and, N170 (150–200 ms) and N300 (275–325 ms) in Left and Right Lateral and Left and Right Semi-Medial regions. The N170 is of particular relevance because previous research has shown that modulation of this component over posterior occipito-temporal areas is sensitive to faces (vs objects; for a review see; Eimer, 2011) and eye gaze direction (e.g. Schweinberger et al., 2007) and therefore, may be more sensitive to effects to specific to eye gaze. Second, temporal principal component analyses (temporal PCA) was conducted to test whether the selected components would emerge as orthogonal components using a data-driven analysis strategy. Temporal PCA takes into account all the variance within selected time window and allows ERP patterns to emerge on the basis of the covariance matrix of the sampled data points. The PCA data set consisted of the ERP averages at each electrode site in all the sampled data (62 electrode sites × 30 participants × experimental conditions) from 0 to 500 ms after the cue onset. The PCA used the covariance matrix with Varimax rotation. Twelve rotated components were extracted (with eigenvalues >1). The first seven components accounted for 91.26% of the variance. The sixth and seventh extracted components are displayed in Figure 2. In agreement with our visual inspection of the grand-averaged ERP waveforms, the sixth component (accounting for 3% of the variance) was similar to the N300, rising at 240 ms to a peak at 300 ms. Furthermore, the fourth component (accounting for 5.22% of the variance) was similar to the N170, rising at 140 ms to a peak at 184 ms ending at 240 ms. This PCA analysis provides confirmatory evidence that the N170 and the N300 are likely to reflect at least partially separable processes.
Fig. 2.
The component waveforms of the extracted principal component analysis (PCA) components. Note that the number of PCA components after Varimax rotation was 12 (with eigenvalues >1), but we have displayed the components that were selected for further analyses. Note that component here refers to PCA component, not to voltage shifts.
RESULTS
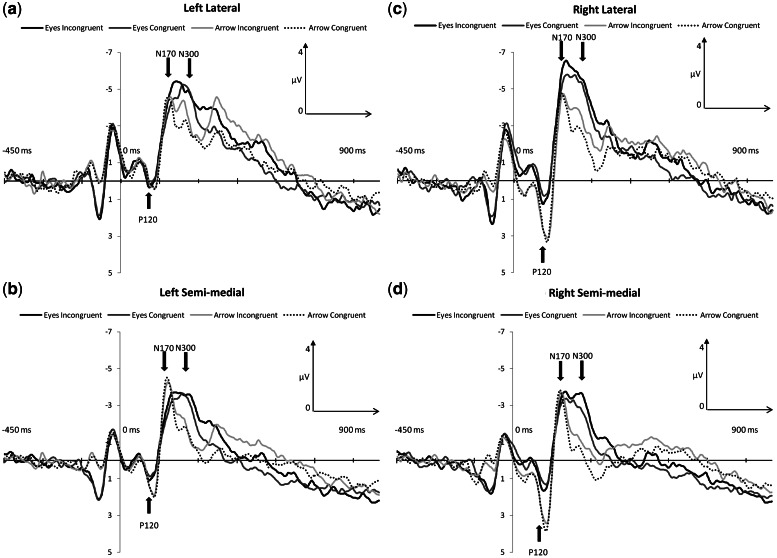
Three ANOVAS were performed to examine effects of interest across the three ERP components P120, N170 and N300. An alpha-level of P < 0.01 for statistical significance was adopted for the results of the initial omnibus ANOVA. For simple main effect analyses of significant interaction effects, an alpha-level of P < 0.05 was used. Figure 3 illustrates grand average ERPs at selected regions of interest.
Fig. 3.
Grand-average ERP waveforms as a function of stimulus type (eyes, arrow) and congruency (congruent or incongruent) are displayed for left lateral (A), left semi-medial (B), right lateral (C) and right semi-medial (D) channel groups.
P120
The mean amplitudes of the Lower Occipital P120 in the test conditions were subjected to a two-way ANOVA with stimulus type (eyes, arrow) and congruency (congruent or incongruent) as within-subject factors. The main effect of stimulus type approached significance, [F (1, 29) = 4.27, P = 0.04,  = 0.12]; the P120 was larger for arrow cues (M = 1.86) than eye gaze cues (M = 1.29). Neither the main effect of congruency not the interaction between congruency and stimulus type were significant (both P > 0.1).
= 0.12]; the P120 was larger for arrow cues (M = 1.86) than eye gaze cues (M = 1.29). Neither the main effect of congruency not the interaction between congruency and stimulus type were significant (both P > 0.1).
N170 and N300
The mean amplitudes of the posterior N170 and N300 in the experimental conditions were subjected to two separate four-way ANOVAs with stimulus type (eyes, arrow), congruency (congruent, incongruent), hemisphere (left, right) and laterality (semi-medial, lateral) as within-subject factors.
N170
In addition to a main effect of laterality, [F (1, 29) = 24.97, P < 0.001, 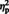 = 0.46], there was a two-way interaction between stimulus type and hemisphere, [F (1, 29) = 12.25, P = 0.002,
= 0.46], there was a two-way interaction between stimulus type and hemisphere, [F (1, 29) = 12.25, P = 0.002, 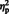 = 0.29]. Simple main effect analyses revealed that the mean N170 elicited to eyes was larger in the right hemisphere (M = −3.38) compared to the N170 elicited to eyes in the left hemisphere (M = −2.76), [F (1, 29) = 5.48, P = 0.02]. For arrows, the N170 did not differ in magnitude between the hemispheres, [F (1, 29) = 2.66, P = 0.08].
= 0.29]. Simple main effect analyses revealed that the mean N170 elicited to eyes was larger in the right hemisphere (M = −3.38) compared to the N170 elicited to eyes in the left hemisphere (M = −2.76), [F (1, 29) = 5.48, P = 0.02]. For arrows, the N170 did not differ in magnitude between the hemispheres, [F (1, 29) = 2.66, P = 0.08].
For the N170, the main effect of laterality was qualified by stimulus type in the form of a stimulus type × laterality interaction, [F (1, 29) = 26.50, P = 0.007, 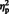 = 0.48]. The simple main effect of laterality was significant for eyes, [F (1, 29) = 67.57, P < 0.0001,
= 0.48]. The simple main effect of laterality was significant for eyes, [F (1, 29) = 67.57, P < 0.0001, 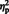 = 0.70] but only marginally so for arrows, [F (1, 29) = 4.15, P = 0.051,
= 0.70] but only marginally so for arrows, [F (1, 29) = 4.15, P = 0.051, 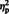 = 0.12]: for eyes, the N170 was larger at lateral electrodes (M = −3.04) compared to semi-medial electrodes (M = −2.53). All other effects failed to reach statistical significance (smallest P = 0.06).
= 0.12]: for eyes, the N170 was larger at lateral electrodes (M = −3.04) compared to semi-medial electrodes (M = −2.53). All other effects failed to reach statistical significance (smallest P = 0.06).
N300
The main effect of congruency, [F (1, 29) = 7.80, P = 0.009, 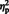 = 0.21] showed that mean N300 amplitude was larger on incongruent (M = −2.48) compared to congruent trials (M = −1.3). The congruency effect (mean incongruent amplitude—mean congruent amplitude) did not differ between eye gaze and arrow trials, [F (1, 29) = 0.88, P = 0.35,
= 0.21] showed that mean N300 amplitude was larger on incongruent (M = −2.48) compared to congruent trials (M = −1.3). The congruency effect (mean incongruent amplitude—mean congruent amplitude) did not differ between eye gaze and arrow trials, [F (1, 29) = 0.88, P = 0.35, 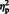 = 0.03]. In addition, the main effect of stimulus type, [F (1, 29) = 15.65, P < 0.001,
= 0.03]. In addition, the main effect of stimulus type, [F (1, 29) = 15.65, P < 0.001, 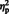 = 0.35] and laterality, [F (1, 29) = 60.16, P < 0.0001,
= 0.35] and laterality, [F (1, 29) = 60.16, P < 0.0001, 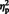 = 0.67] showed (respectively) that the mean N300 was larger for eyes (M = −2.69) compared to arrows (M = −1.17), and also, larger at lateral electrode sites (M = −2.87) compared to semi-medial electrode sites (M = −1.01). A weak main effect of hemisphere, [F (1, 29) = 3.57, P = 0.07,
= 0.67] showed (respectively) that the mean N300 was larger for eyes (M = −2.69) compared to arrows (M = −1.17), and also, larger at lateral electrode sites (M = −2.87) compared to semi-medial electrode sites (M = −1.01). A weak main effect of hemisphere, [F (1, 29) = 3.57, P = 0.07, 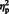 = 0.11] was qualified by a stimulus type × hemisphere interaction, [F (1, 29) = 8.07, P = 0.008,
= 0.11] was qualified by a stimulus type × hemisphere interaction, [F (1, 29) = 8.07, P = 0.008, 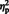 = 0.22]. Simple main effect analyses showed that for arrows, [F (1, 29) = 5.80, P = 0.01,
= 0.22]. Simple main effect analyses showed that for arrows, [F (1, 29) = 5.80, P = 0.01, 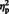 = 0.17] but not eyes, [F (1, 29) = 0.35, P = 0.55,
= 0.17] but not eyes, [F (1, 29) = 0.35, P = 0.55, 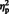 < 0.05], the N300 was smaller in the right (M = −1.24, s.d. = 2.99) compared to the left hemisphere (M = −2.15). All other effects failed to reach statistical significance (smallest P = 0.08).
< 0.05], the N300 was smaller in the right (M = −1.24, s.d. = 2.99) compared to the left hemisphere (M = −2.15). All other effects failed to reach statistical significance (smallest P = 0.08).
DISCUSSION
Following recent research, there was an enhanced mid-latency negative component (N300) when participants viewed a person looking away from a location previously cued by a flashing checkerboard, compared to viewing a person looking towards a location previously occupied by a checkerboard. We consider our enhanced negative component for object-incongruent relative to object-congruent shifts to be equivalent to the enhanced negative component (N330) reported by Senju et al. (2006). In that research, modulation of the negative component was interpreted as brain activity that reflects a basic process whereby individuals generate expectations of rational behavior of other individuals in the context of specific events. Here, the new finding is that the enhanced negative component was recorded for a non-human cue (an arrow) on object-incongruent trials and therefore, we conclude that enhanced negativity in response to violations of expectation occurs more generally for both human and non-human cues.
We interpret these effects in the context of domain-general computational mechanism for detecting salient, unexpected events described by Corbetta et al. (2008). They summarize research studies that have reported consistent activation in a ventral frontoparietal network that includes regions that adjoin the STS such as the temporoparietal junction. For example, expectancy was manipulated in one study (Corbetta et al., 2000) by comparing brain activation on trials on which targets appeared at an expected location (indicated by an arrow) with trials on which the target appeared at an unexpected location. The authors recorded increased activation in the right Temporal Parietal Junction (rTPJ) for targets appearing at unexpected locations compared to targets appearing at expected locations. As noted earlier, a recent study has shown that, compared to orienting to non-social cues, distinct patterns of activation in the rTPJ are found when individuals carryout a Theory of Mind task. Further fMRI research is needed to examine whether movement of the eyes to unexpected locations and other forms of human movement to unexpected locations used by Pelphrey et al. (2003) activate distinct regions of the TPJ and STS compared to the non-social direction cues used in the current research. Although the current findings suggest a shared mechanism instantiated in the same region of the brain, fMRI with similar high spatial resolution to that used recently (Scholz et al., 2009) may help locate distinct regions of activation for unexpected movement of the eyes compared to non-human symbolic cues such as arrows.
Although the modulation of the N300 due to object-congruency did not differ between eye gaze and arrow cues, stimuli type modulated the N170 and N300 in keeping with the idea that face stimuli engage specialized processes. Following previous studies (Bentin et al., 1996; Carmel and Bentin, 2002; Jacques and Rossion, 2007; Landau et al., 2010), the enhanced N170 to eye gaze (face stimulus) was larger in the right compared to the left hemisphere. Similarly, and in keeping with the idea of right hemisphere specialization for face stimuli, the N300 was smaller in the right compared to the left for arrows but not eyes. Right hemisphere specialization for eye gaze but not arrows is consistent with fMRI research that has consistently found increased activity in the right fusiform gyrus for face stimuli compared to other categories of stimuli and also, scrambled face images (for a review see; McKone et al., 2007). Modulation of the N170 for eye gaze stimuli is important because it shows that although there was no difference in the object-congruency effect between gaze and arrows, our task was sensitive to theoretically relevant differences between stimuli. In others words, we were able to record effects unique to eyes even though such effects were not found in terms of differences in expected movement.
The findings reported here differ from those reported by Senju et al. (2006) in a number of ways. First, the mean amplitude of the enhanced negative ERP for object-incongruent shifts occurred earlier in the current study (at N300 rather than N330). The negative deflection may have been earlier in the current study because we used a single face stimulus, whereas Senju et al. (2006) used multiple stimuli that varied in identity and other facial characteristics that may have reduced the speed of face processing for their stimuli relative to the stimuli used in the current study. Second, in the current research we recorded larger mean amplitudes at the P120 but this effect did not vary as a function of object-congruency. Nonetheless, there was a trend for the modulation of the P120 due to the type of stimulus displayed—it was larger for arrow cues. The increased P120 for arrow cues may reflect the greater visual differences between the fixation stimulus and subsequent averted arrow cue compared to the fixation and averted gaze cue; for the arrow cue trials the entire fixation cue was replaced (albeit with a visually similar stimulus), whereas for gaze cue trials the gaze simply changed location (from direct to averted). In short, the larger P120 for arrow cues may have reflected greater early visual processing for the arrow compared to the face stimuli trials.
As noted in the introduction, the effects reported here and those of Senju et al. (2006) relate to a specific type of expectation—expected movement to a specific event. A separate line of research of research has studied the neural processes responsible for goal and trait inference (Van Duynslaeger et al., 2007; Van der Cruyssen et al., 2009) and more specifically, the effects of expectancy-violating behavioral information (Bartholow et al., 2001). For example, Bartholow et al. (2001) examined the electrophysiological correlates of a classic effect from social psychology namely, enhanced recall for expectancy-violating behavioral information (for reviews see; Stangor and McMillan, 1992; Ybarra, 2002). The recall advantage is thought to reflect updating in working memory (Srull and Wyer, 1989) and therefore, given the use of the P300 as an index of working memory updating (e.g. Donchin and Coles, 1988) and social cognitive processes such as evaluative categorization (e.g. Cacioppo et al., 1994), Bartholow et al. (2001) tested the idea that expectancy-violating behaviors would modulate the P300. In support, they recorded an amplified P300 when individuals read descriptions of (unexpected) behavior that were inconsistent with an impression of an individual they had formed earlier. One potentially fruitful approach would be to combine the study of eye gaze and arrow cues adopted here with the study of trait inference (for similar approach with eye gaze cues see; MacRae et al., 2002) to provide a test of the idea that specialized neural processes operate in response to eye gaze cues during the processing of trait related information.
In conclusion, the current findings offer preliminary support for the idea that violations of expectation from observing gaze shifts reflect the operation of a domain general computation mechanism that also operates in response to non-social stimuli.
FUNDING
This work was supported by Economic Social Research Council grant (RES-000-22-2529) awarded to Jason Tipples.
REFERENCES
- Baron-Cohen S. Mindblindness. Cambridge, MA: MIT Press/Bradford; 1995. [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, O’Riordan M, Stone V, Jones R, Plaisted K. Recognition of faux pas by normally developing children and children with Asperger syndrome or high-functioning autism. Journal of Autism and Developmental Disorders. 1999;29(5):407–18. doi: 10.1023/a:1023035012436. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Fabiani M, Gratton G, Bettencourt BA. A psychophysiological analysis of cognitive processing of and affective responses to social expectancy violations. Psychological Science. 2001;12:197–204. doi: 10.1111/1467-9280.00336. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–65. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Schmitt RM, Spengler S, Gergely G. Investigating action understanding: inferential processes versus action simulation. Current Biology. 2007;17(24):2117–21. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Crites SL, Jr, Gardner WL, Berntson GG. Bioelectrical echoes from evaluative categorizations: I. A late positive brain potential that varies as a function of trait negativity and extremity. Journal of Personality and Social Psychology. 1994;67:115–25. doi: 10.1037//0022-3514.67.1.115. [DOI] [PubMed] [Google Scholar]
- Carmel D, Bentin S. Domain specificity versus expertise: factors influencing distinct processing of faces. Cognition. 2002;83(1):1–29. doi: 10.1016/s0010-0277(01)00162-7. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3(3):292–7. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennett DC. The Intentional Stance. Cambridge, MA.: MIT Press; 1987. [Google Scholar]
- Donchin E, Coles MG. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–427. [Google Scholar]
- Eimer M. The face-sensitive N170 component of the event-related brain potential. In: Calder AJ, Rhodes G, Johnson M, Haxby J, editors. The Oxford Handbook of Face Perception. Oxford: Oxford University Press; 2011. pp. 329–44. [Google Scholar]
- Engell AD, Nummenmaa L, Oosterhof NN, Henson RN, Haxby JV, Calder AJ. Differential activation of frontoparietal attention networks by social and symbolic spatial cues. Social, Cognitive and Affective Neuroscience. 2010;5:432–40. doi: 10.1093/scan/nsq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Colich N, Iacoboni M, Zaidel E, Bookheimer SY, Dapretto M. Atypical neural networks for social orienting in autism spectrum disorders. Neuroimage. 2011;56:354–62. doi: 10.1016/j.neuroimage.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Mooshagian E, Kaplan JT, Zaidel E, Iacoboni M. The neural correlates of social attention: automatic orienting to social and nonsocial cues. Psychological Research. 2009;73:499–511. doi: 10.1007/s00426-009-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grezes J, Frith C, Passingham RE. Brain mechanisms for inferring deceit in the actions of others. Journal of Neuroscience. 2004;24(24):5500–5. doi: 10.1523/JNEUROSCI.0219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques C, Rossion B. Electrophysiological evidence for temporal dissociation between spatial attention and sensory competition during human face processing. Cerebral Cortex. 2007;17(5):1055–65. doi: 10.1093/cercor/bhl015. [DOI] [PubMed] [Google Scholar]
- Jastorff J, Clavagnier S, Gergely G, Orban GA. Neural mechanisms of understanding rational actions: middle temporal gyrus activation by contextual violation. Cerebral Cortex. 2011;21(2):318–29. doi: 10.1093/cercor/bhq098. [DOI] [PubMed] [Google Scholar]
- Landau AN, Aziz-Zadeh L, Ivry RB. The influence of language on perception: listening to sentences about faces affects the perception of faces. The Journal of Neuroscience. 2010;30(45):15254–61. doi: 10.1523/JNEUROSCI.2046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam SR, Hunnisett E, Moore C. Targets and cues: gaze-following in children with autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39(7):951–62. [PubMed] [Google Scholar]
- Leekam SR, Lopez B, Moore C. Attention and joint attention in preschool children with autism. Developmental Psychology. 2000;36(2):261–73. doi: 10.1037//0012-1649.36.2.261. [DOI] [PubMed] [Google Scholar]
- MacRae CN, Hood BM, Milne AB, Rowe AC, Mason MF. Are you looking at me?: Eye gaze and person perception. Psychological Science. 2002;13:460–4. doi: 10.1111/1467-9280.00481. [DOI] [PubMed] [Google Scholar]
- McKone E, Kanwisher N, Duchaine BC. Can generic expertise explain special processing for faces? Trends in Cognitive Sciences. 2007;11(1):8–15. doi: 10.1016/j.tics.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cerebral Cortex. 2007;18:262–71. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Forming impressions of people versus inanimate objects: social-cognitive processing in the medial prefrontal cortex. Neuroimage. 2005;26:251–7. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Regional brain activation evoked when approaching a virtual human on a virtual walk. Journal of Cognitive Neuroscience. 2005;17(11):1744–52. doi: 10.1162/089892905774589253. [DOI] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Occipitotemporal activation evoked by the perception of human bodies is modulated by the presence or absence of the face. Neuropsychologia. 2006;44:1919–27. doi: 10.1016/j.neuropsychologia.2006.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Mack PB, McCarthy G, Pelphrey KA. Taking an “intentional stance” on eye-gaze shifts: a functional neuroimaging study of social perception in children. Neuroimage. 2005;27(1):247–52. doi: 10.1016/j.neuroimage.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Grasping the intentions of others: the perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. Journal of Cognitive Neuroscience. 2004;16(10):1706–16. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005a;128:1038–48. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G. Functional anatomy of biological motion perception in posterior temporal cortex: an fMRI study of eye, mouth and hand movements. Cerebral Cortex. 2005b;15(12):1866–76. doi: 10.1093/cercor/bhi064. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G. Brain activation evoked by perception of gaze shifts; the influence of context. Neuropsychologia. 2003;41(2):156–70. doi: 10.1016/s0028-3932(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. Journal of Neuroscience. 1998;18(6):2188–99. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Perrett D. Electrophysiology and brain imaging of biological motion. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2003;358(1431):435–45. doi: 10.1098/rstb.2002.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic J, Friesen CK, Kingstone A. Are eyes special? It depends on how you look at it. Psychonomic Bulletin & Review. 2002;9(3):507–13. doi: 10.3758/bf03196306. [DOI] [PubMed] [Google Scholar]
- Saxe R, Xiao DK, Kovacs G, Perrett DI, Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42(11):1435–46. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Saygin AP, Wilson SM, Hagler DJ, Bates E, Sereno MI. Point-light biological motion perception activates human premotor cortex. Journal of Neuroscience. 2004;24(27):6181–8. doi: 10.1523/JNEUROSCI.0504-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, Saxe R. Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS ONE. 2009;4:e4869. doi: 10.1371/journal.pone.0004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller AM, Rossion B. Spatial attention triggered by eye gaze increases and speeds up early visual activity. Neuroreport. 2001;12(11):2381–6. doi: 10.1097/00001756-200108080-00019. [DOI] [PubMed] [Google Scholar]
- Schweinberger SR, Kloth N, Jenkins R. Are you looking at me? Neural correlates of gaze adaptation. Neuroreport. 2007;18:693–6. doi: 10.1097/WNR.0b013e3280c1e2d2. [DOI] [PubMed] [Google Scholar]
- Senju A, Johnson MH, Csibra G. The development and neural basis of referential gaze perception. Social Neuroscience. 2006;1(3–4):220–34. doi: 10.1080/17470910600989797. [DOI] [PubMed] [Google Scholar]
- Srull TK, Wyer RS. Person memory and judgment. Psychological Review. 1989;96:58–83. doi: 10.1037/0033-295x.96.1.58. [DOI] [PubMed] [Google Scholar]
- Stangor C, McMillan D. Memory for expectancy-congruent and expectancy-incongruent information: a review of the social and social-developmental literatures. Psychological Bulletin. 1992;111:42–61. [Google Scholar]
- Teufel C, Alexis DM, Clayton N, Davis G. Mental-state attribution drives rapid, reflexive gaze following. Attention, Perception, & Psychophysics. 2010;72:695–705. doi: 10.3758/APP.72.3.695. [DOI] [PubMed] [Google Scholar]
- Tipples J. Eye gaze is not unique: automatic orienting in response to uninformative arrows. Psychonomic Bulletin & Review. 2002;9(2):314–8. doi: 10.3758/bf03196287. [DOI] [PubMed] [Google Scholar]
- Van der Cruyssen L, Van Duynslaeger M, Cortoos A, Van Overwalle F. ERP time course and brain areas of spontaneous and intentional goal inferences. Social Neuroscience. 2009;4:165–84. doi: 10.1080/17470910802253836. [DOI] [PubMed] [Google Scholar]
- Van Duynslaeger M, Van Overwalle F, Verstraeten E. Electrophysiological time course and brain areas of spontaneous and intentional trait inferences. Social Cognitive and Affective Neuroscience. 2007;2:174–88. doi: 10.1093/scan/nsm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyk BCV, Hudac CM, Carter EJ, Sobel DM, Pelphrey KA. Action understanding in the superior temporal sulcus region. Psychological Science. 2009;20(6):771–7. doi: 10.1111/j.1467-9280.2009.02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybarra O. Naive causal understanding of valenced behaviors and its implications for social information processing. Psychological Bulletin. 2002;128:421–41. doi: 10.1037/0033-2909.128.3.421. [DOI] [PubMed] [Google Scholar]