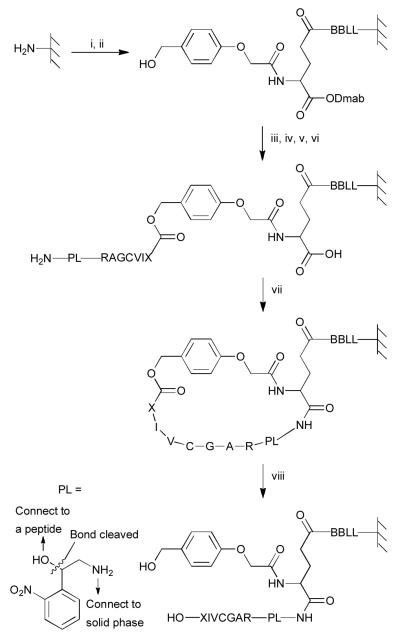
Fig. 2.
Synthesis of C-terminal peptides exemplified by a RAGCVIX library. Reagents and conditions: (i) DIC coupling of Fmoc-Aa (2×), then capping, then 20% piperidine, then BPB staining; (ii) DIC coupling of HMPA (2×); (iii) 0.5 M Fmoc-Aa and 0.5 M 6-Cl-HOBt in DMF/CH2Cl2, 0.5 M DIC in CH2Cl2, 0.2 M DMAP in CH2Cl2 (6-8×), then capping, then 20% piperidine, then BPB staining; (iv) DIC coupling of Fmoc-Aa (2×), then capping, then 20% piperidine, then BPB staining; (v) 0.5 M photolysis linker, 0.5 M Et3N in DMF (3-4×); (vi) 2% hydrazine; (vii) 0.05 M BOP, 0.05 M 6-Cl-HOBt and 0.1 M DIEA in DMF (2×); (viii) modified reagent K. PL represents a cleavable linker so that peptides can be cleaved from resin and analyzed by MS. B represents β-alanine.