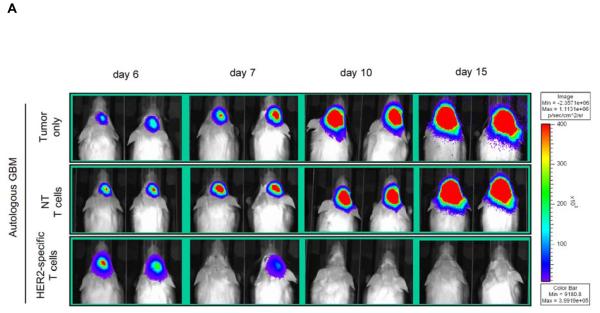
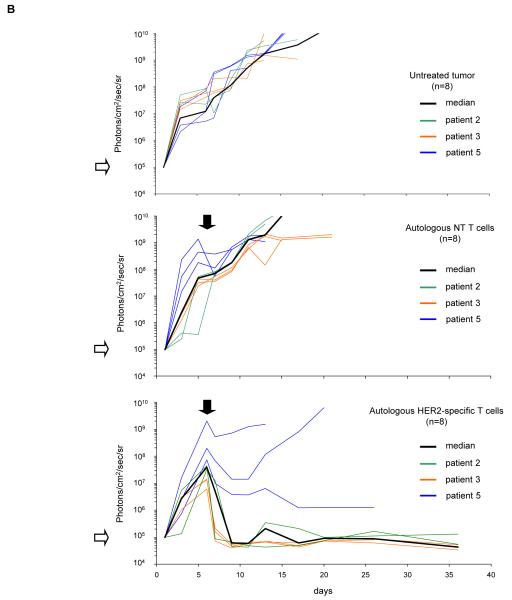
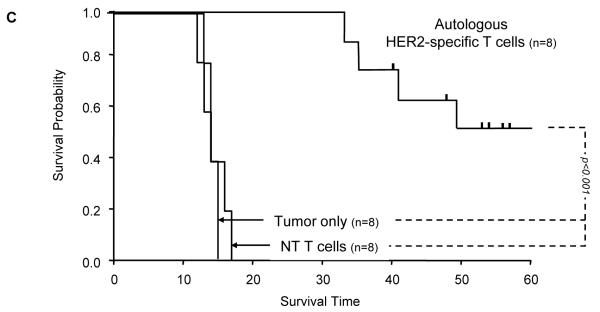
Figure 5. Adoptively transferred HER2-specific T cells induce regression of autologous GBM xenografts in vivo.
5×104 primary GBM cells from patients 2 (2 mice per group), 3 (3 mice per group) and 5 (3 mice per group) were injected stereotactically into the caudate nucleus of 9 to 12 week old SCID mice followed by intratumoral injection of 2×106 autologous HER2-specific or non-transduced T cells (NT-T cells) 6 days after tumor inoculation. (A) Tumors grew progressively in untreated mice as shown for two representative animals (upper row) and in mice receiving non-transduced T cells (middle row), while tumors regressed over a period of 2-5 days in response to a single injection of autologous HER2-specific T cells generated from the same patient (lower row). (B) Quantitative bioluminescence imaging: Autologous HER2-specific T cells induced tumor regression when compared to NT-T cells (two-tailed P value = 0.002, Mann-Whitney U test). Solid arrows: time of T-cell injection; open arrows: background luminescence (mean~105 photon/sec/cm2/sr); n=number of animals tested in each group. (C) Kaplan-Meier survival curve: Survival analysis performed 60 days after tumor establishment. Mice treated with autologous HER2-specific T cells had a significantly longer survival probability (p<0.001) in comparison to untreated mice and mice that received NT-T cells. (D) 1×104 CD133-positive GBM cells from patient 2 were injected as described above followed by intratumoral injection of 2×106 autologous HER2-specific or NT-T cells 8 days after tumor inoculation. While tumors in animals treated with NT T cells (n=4) continued to grow exponentially, all of the animals treated with autologous HER2 T cell (n=4) regressed with two of these animals having no detectable tumors with in 6 days after T-cell injection.