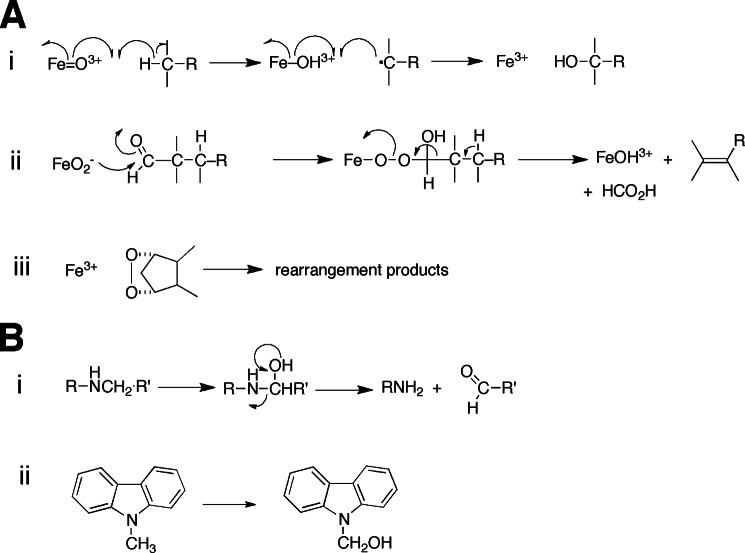
FIGURE 1.
A, major modes of oxidation reactions catalyzed by P450 enzymes. i, Compound I (FeO3+) with hydrogen abstraction and oxygen rebound. A variant on this is the initial abstraction of a non-bonded electron from a heteroatom, followed by base-catalyzed rearrangement of the aminium radical (N+·) to a carbon radical prior to oxygen rebound (2, 3). ii, reaction of an iron peroxy anion (normally an intermediate in the production of FeO3+) with an aldehyde, probably the best documented example of this kind of chemistry (4). The process leads to an alkene or aromatic ring, e.g. estrogen synthesis in the aromatase (CYP19A1) reaction (see Fig. 2B). iii, rearrangement of an oxidized entity, exemplified here by the P450 CYP58A1 formation of TXA2 from PGH2. B, rearrangements. i, formation and non-enzymatic rearrangement of a carbinolamine and a gem-halohydrin. ii, a stable carbinolamine formed from N-methylcarbazole in P450 oxidations (5, 6).