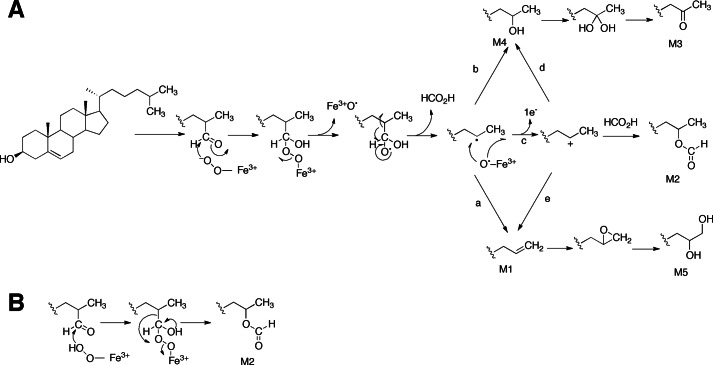
FIGURE 2.
Possible mechanisms for steroid deformylation reactions catalyzed by the P450 CYP125. A, the important steps involve addition of the ferric-peroxo anion (FeO2−) of CYP125 to the C-26 carbonyl and subsequent radical fragmentation of the peroxyhemiacetal adduct (27). The radical fragmentation of the peroxyhemiacetal adduct leads to formation of an alkene (M1; arrow a) or a one-carbon deficient alcohol (M4; arrow b). The Compound I-catalyzed oxidation of M1 generates a diol (M5) via the acid-catalyzed ring opening of an epoxide intermediate. A C-25 cation may also derive from the single-electron oxidation of the C-25 radical (arrow c). Trapping of the cation by formate or water (arrow d) results in the formation of the C-25 oxyformyl (M2) or the one-carbon reduced alcohol (M4), respectively. Loss of a proton from the C-25 cation may also generate M1 (arrow e). The Compound I-catalyzed oxidation of M4 produces a gem-diol intermediate that dehydrates to a keto compound (M3). B, a possible alternative mechanism involving Baeyer-Villiger oxidation with the ferric-peroxo anion (FeO2−) of CYP125 to yield M2 (see Fig. 1B, ii).