FIGURE 1.
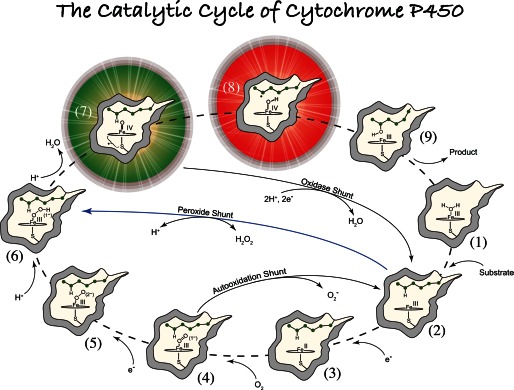
General paradigm for P450-catalyzed hydroxylations. The first step involves the binding of substrate to the resting low-spin ferric enzyme (1). This binding induces structural changes, which often, but not always, manifest themselves in the dissociation of the distally coordinated water and the conversion of the heme from low to high spin (2). These substrate-induced structural changes facilitate reduction of the ferric enzyme, allowing delivery of the first electron to generate the ferrous substrate-bound form of the enzyme (3). Dioxygen then binds to the ferrous heme, forming a species that is best described as a ferric superoxide complex (4). The subsequent reduction of this species forms a ferric peroxo species (5), which is protonated at the distal oxygen to generate a ferric hydroperoxo complex (6). The delivery of an additional proton to the distal oxygen cleaves the O–O bond, yielding compound I (7) and a water molecule. Compound I then abstracts hydrogen from substrate to yield compound II (8) and a substrate radical, which rapidly recombine to yield hydroxylated product and ferric enzyme (9). Hydroxylated product then dissociates, and water coordinates to the heme to regenerate the resting ferric enzyme (1).
