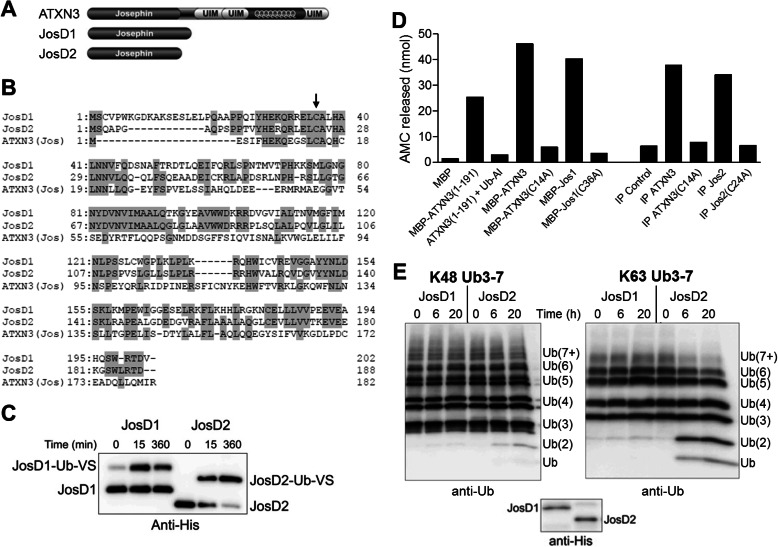
FIGURE 1.
JosD1 and JosD2 differ in DUB activity in vitro. A, domain structure composition of ATXN3, JosD1, and JosD2. In addition to the Josephin domain, ATXN3 has three UIMs flanking a polyglutamine repeat. B, sequence comparison of JosD1, JosD2, and the Josephin domain of ATXN3. Amino acid residues conserved between two or more proteins are highlighted with gray. Arrow indicates the cysteine residue essential for catalytic activity (JosD1, Cys36; JosD2, Cys24; ATXN3, Cys14). C, catalytic activity of JosD1 and JosD2 assessed by reaction with Ub-VS. His-tagged JosD1 and JosD2 were incubated with Ub-VS for the indicated times and detected by immunoblotting with anti-His tag antibody. Ub-VS-modified JosD1 and JosD2 electrophorese at higher molecular mass. D, DUB activity of ATXN3, JosD1, and JosD2 determined by Ub-AMC. DUB activity was measured by fluorescence AMC released from Ub-AMC. Recombinant JosD1 fused to maltose-binding protein (MBP) has DUB activity similar to full-length ATXN3 and the isolated Josephin domain (1–191) of ATXN3. Immunoprecipitated (IP) JosD2 also has DUB activity. DUB activity of ATXN3, JosD1, and JosD2 is eliminated by mutating the catalytic cysteine residue (Cys14 in ATXN3, Cys36 in JosD1, and Cys24 in JosD2). E, divergent DUB activity of JosD1 and JosD2 assessed in vitro by cleavage of polyubiquitin chains. His-tagged JosD1 and JosD2 were incubated with Lys48-linked (left) or Lys63-linked (right) poly-Ub chains (3–7 Ubs in length) for the indicated times. Ub chains were detected by immunoblotting with anti-ubiquitin antibody. Lower immunoblot shows the amount of JosD1 and JosD2 used in the assay, detected with anti-His antibody. Whereas JosD2 cleaves poly-Ub chains, JosD1 shows little to no cleavage.