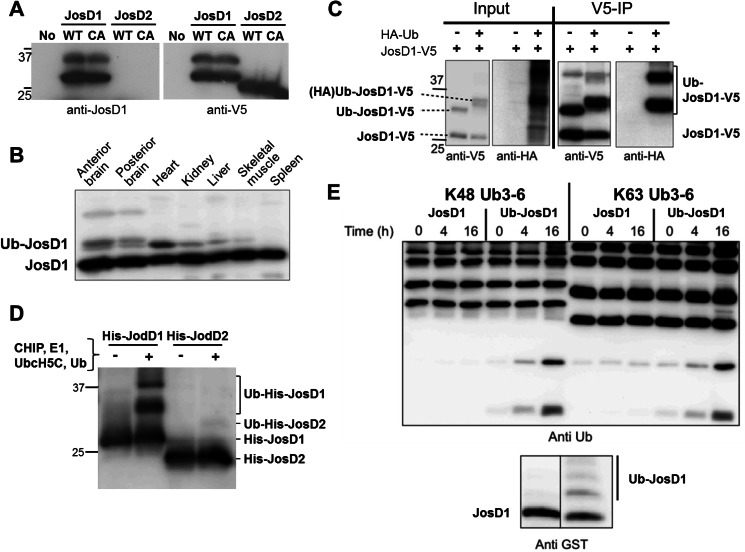
FIGURE 2.
Ubiquitination of JosD1 enhances DUB activity. A, specificity of generated anti-JosD1 antibody. JosD1-V5 (wild-type (WT) and C36A (CA) mutant) and JosD2 (WT and C24A (CA) mutant), when transiently expressed in HEK-293 cells, were detected by immunoblotting with anti-JosD1 (left) and anti-V5 (right) antibodies. The JosD1 antibody detected overexpressed JosD1-V5, but not JosD2-V5. This antibody could not detect endogenous JosD1 in HEK-293 cells. B, JosD1 expression in mouse tissues. Protein lysates from the indicated tissues were immunoblotted with anti-JosD1 antibody. A strong band is seen in all tissues, with some tissues also showing a higher molecular mass band consistent with monoubiquitinated JosD1 (Ub-JosD1). C, confirmation of JosD1 monoubiquitination cells. JosD1-V5 and HA-Ub were co-expressed in COS-7 cells, and cell lysates were immunoprecipitated (IP) with anti-V5 antibody. HA-ubiquitinated JosD1 was strongly detected in immunoprecipitates. D, ubiquitination of JosD1 in vitro. Recombinant His-JosD1 and His-JosD2 were incubated with CHIP, UbcH5c, E1, and Ub to drive ubiquitination. JosD1, but not JosD2, is ubiquitinated in these reactions. E, ubiquitination of JosD1 enhances DUB activity in vitro. GST-JosD1 and ubiquitinated GST-JosD1 (Ub-JosD1) were incubated with Lys48-linked (left) or Lys63-linked (right) poly-Ub chains for the indicated periods of time. Lower immunoblot shows the levels of JosD1 and Ub-JosD1 used in the assay, detected with anti-GST antibody.