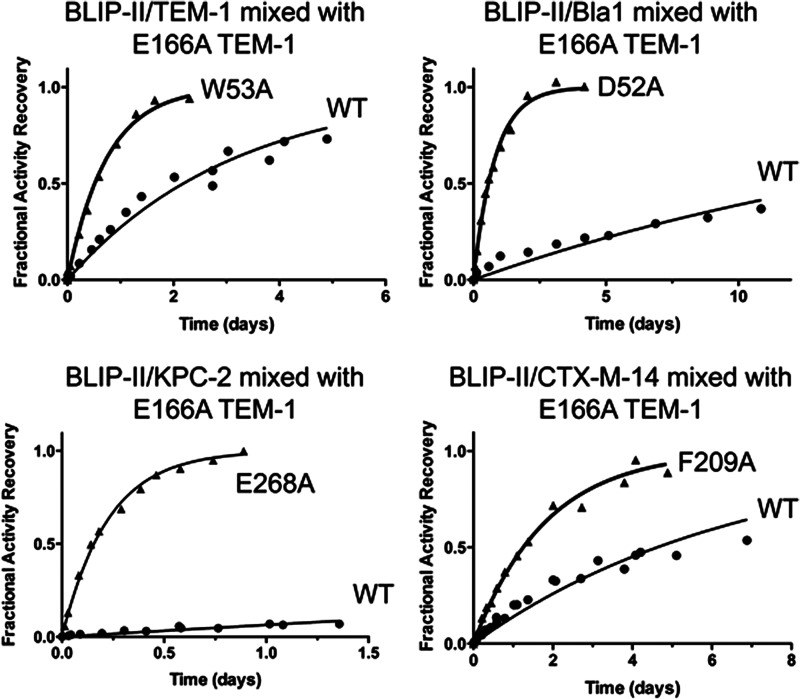
FIGURE 3.
Enzymatic activity-based measurements of dissociation. Plots of the dissociation reactions between BLIP-II (wild type or alanine variants) and β-lactamase are shown. The fractional recovered enzymatic activities of TEM-1(2.5 nm complex, top left), Bla1 (50 nm complex, top right), KPC-2 (50 nm complex, bottom left), and CTX-M-14 (10 nm complex, bottom right) are plotted versus time, t, after mixing of the corresponding BLIP-II-β-lactamase complexes with ≥200 molar excess of inactive, deacylation deficient TEM-1 variant, E166A, competitor to absorb free BLIP-II. The solid circles are the normalized recovered activities from the corresponding wild-type complexes, and the solid triangles are from the labeled mutant complexes, whereas the solid curves are the fitting curves of the first order kinetics according Equation 7 (see “Experimental Procedures”) to determine the appropriate koff values (tabulated in Table 1). The experiments to determine the dissociation rate constants were performed in triplicate, and all of the data sets were combined and used to obtain the rate constant with an associated standard error through nonlinear regression.